Structure of a novel phosphotyrosine-binding domain in Hakai that targets E-cadherin
- PMID: 22252131
- PMCID: PMC3298002
- DOI: 10.1038/emboj.2011.496
Structure of a novel phosphotyrosine-binding domain in Hakai that targets E-cadherin
Abstract
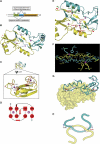
Phosphotyrosine-binding domains, typified by the SH2 (Src homology 2) and PTB domains, are critical upstream components of signal transduction pathways. The E3 ubiquitin ligase Hakai targets tyrosine-phosphorylated E-cadherin via an uncharacterized domain. In this study, the crystal structure of Hakai (amino acids 106-206) revealed that it forms an atypical, zinc-coordinated homodimer by utilizing residues from the phosphotyrosine-binding domain of two Hakai monomers. Hakai dimerization allows the formation of a phosphotyrosine-binding pocket that recognizes specific phosphorylated tyrosines and flanking acidic amino acids of Src substrates, such as E-cadherin, cortactin and DOK1. NMR and mutational analysis identified the Hakai residues required for target binding within the binding pocket, now named the HYB domain. ZNF645 also possesses a HYB domain but demonstrates different target specificities. The HYB domain is structurally different from other phosphotyrosine-binding domains and is a potential drug target due to its novel structural features.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures






References
-
- Adams PD, Grosse-Kunstleve RW, Hung LW, Ioerger TR, McCoy AJ, Moriarty NW, Read RJ, Sacchettini JC, Sauter NK, Terwilliger TC (2002) PHENIX: building new software for automated crystallographic structure determination. Acta Crystallogr D Biol Crystallogr 58: 1948–1954 - PubMed
-
- Bellon SF, Rodgers KK, Schatz DG, Coleman JE, Steitz TA (1997) Crystal structure of the RAG1 dimerization domain reveals multiple zinc-binding motifs including a novel zinc binuclear cluster. Nat Struct Biol 4: 586–591 - PubMed
-
- Benes CH, Wu N, Elia AE, Dharia T, Cantley LC, Soltoff SP (2005) The C2 domain of PKCdelta is a phosphotyrosine binding domain. Cell 121: 271–280 - PubMed
-
- Borden KLB (2000) RING domains: master builders of molecular scaffolds? J Mol Biol 295: 1103–1112 - PubMed
-
- Brunger AT, Adams PD, Clore GM, DeLano WL, Gros P, Grosse-Kunstleve RW, Jiang JS, Kuszewski J, Nilges M, Pannu NS, Read RJ, Rice LM, Simonson T, Warren GL (1998) Crystallography & NMR system: a new software suite for macromolecular structure determination. Acta Crystallogr D Biol Crystallogr 54: 905–921 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

