Using PET with 18F-AV-45 (florbetapir) to quantify brain amyloid load in a clinical environment
- PMID: 22252372
- PMCID: PMC3315642
- DOI: 10.1007/s00259-011-2021-8
Using PET with 18F-AV-45 (florbetapir) to quantify brain amyloid load in a clinical environment
Abstract
Purpose: Positron emission tomography (PET) imaging of brain amyloid load has been suggested as a core biomarker for Alzheimer's disease (AD). The aim of this study was to test the feasibility of using PET imaging with (18)F-AV-45 (florbetapir) in a routine clinical environment to differentiate between patients with mild to moderate AD and mild cognitive impairment (MCI) from normal healthy controls (HC).
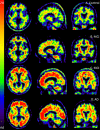
Methods: In this study, 46 subjects (20 men and 26 women, mean age of 69.0 ± 7.6 years), including 13 with AD, 12 with MCI and 21 HC subjects, were enrolled from three academic memory clinics. PET images were acquired over a 10-min period 50 min after injection of florbetapir (mean ± SD of radioactivity injected, 259 ± 57 MBq). PET images were assessed visually by two individuals blinded to any clinical information and quantitatively via the standard uptake value ratio (SUVr) in the specific regions of interest, which were defined in relation to the cerebellum as the reference region.
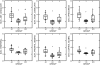
Results: The mean values of SUVr were higher in AD patients (median 1.20, Q1-Q3 1.16-1.30) than in HC subjects (median 1.05, Q1-Q3 1.04-1.08; p = 0.0001) in the overall cortex and all cortical regions (precuneus, anterior and posterior cingulate, and frontal median, temporal, parietal and occipital cortex). The MCI subjects also showed a higher uptake of florbetapir in the posterior cingulate cortex (median 1.06, Q1-Q3 0.97-1.28) compared with HC subjects (median 0.95, Q1-Q3 0.82-1.02; p = 0.03). Qualitative visual assessment of the PET scans showed a sensitivity of 84.6% (95% CI 0.55-0.98) and a specificity of 38.1% (95% CI 0.18-0.62) for discriminating AD patients from HC subjects; however, the quantitative assessment of the global cortex SUVr showed a sensitivity of 92.3% and specificity of 90.5% with a cut-off value of 1.122 (area under the curve 0.894).
Conclusion: These preliminary results suggest that PET with florbetapir is a safe and suitable biomarker for AD that can be used routinely in a clinical environment. However, the low specificity of the visual PET scan assessment could be improved by the use of specific training and automatic or semiautomatic quantification tools.
Figures
Similar articles
-
In vivo imaging of amyloid deposition in Alzheimer disease using the radioligand 18F-AV-45 (florbetapir [corrected] F 18).J Nucl Med. 2010 Jun;51(6):913-20. doi: 10.2967/jnumed.109.069088. J Nucl Med. 2010. PMID: 20501908 Free PMC article. Clinical Trial.
-
Imaging characteristic of dual-phase (18)F-florbetapir (AV-45/Amyvid) PET for the concomitant detection of perfusion deficits and beta-amyloid deposition in Alzheimer's disease and mild cognitive impairment.Eur J Nucl Med Mol Imaging. 2016 Jul;43(7):1304-14. doi: 10.1007/s00259-016-3359-8. Epub 2016 Mar 22. Eur J Nucl Med Mol Imaging. 2016. PMID: 27003417
-
Cognition and amyloid load in Alzheimer disease imaged with florbetapir F 18(AV-45) positron emission tomography.Am J Geriatr Psychiatry. 2013 Mar;21(3):272-8. doi: 10.1016/j.jagp.2012.11.016. Am J Geriatr Psychiatry. 2013. PMID: 23395194 Free PMC article.
-
Florbetapir (18F), a PET imaging agent that binds to amyloid plaques for the potential detection of Alzheimer's disease.IDrugs. 2010 Dec;13(12):890-9. IDrugs. 2010. PMID: 21154149 Review.
-
[Clinical Implications and Appropriate Use of Amyloid Imaging with florbetapir (18F) in Diagnosis of Patients with Alzheimer Disease].Brain Nerve. 2016 Oct;68(10):1215-1222. doi: 10.11477/mf.1416200578. Brain Nerve. 2016. PMID: 27703109 Review. Japanese.
Cited by
-
Longitudinal Positron Emission Tomography in Preventive Alzheimer's Disease Drug Trials, Critical Barriers from Imaging Science Perspective.Brain Pathol. 2016 Sep;26(5):664-71. doi: 10.1111/bpa.12399. Brain Pathol. 2016. PMID: 27327527 Free PMC article.
-
Discovering network phenotype between genetic risk factors and disease status via diagnosis-aligned multi-modality regression method in Alzheimer's disease.Bioinformatics. 2019 Jun 1;35(11):1948-1957. doi: 10.1093/bioinformatics/bty911. Bioinformatics. 2019. PMID: 30395195 Free PMC article.
-
Classification of Alzheimer's Disease, Mild Cognitive Impairment, and Cognitively Unimpaired Individuals Using Multi-feature Kernel Discriminant Dictionary Learning.Front Comput Neurosci. 2018 Jan 9;11:117. doi: 10.3389/fncom.2017.00117. eCollection 2017. Front Comput Neurosci. 2018. PMID: 29375356 Free PMC article.
-
Alzheimer disease: epidemiology, diagnostic criteria, risk factors and biomarkers.Biochem Pharmacol. 2014 Apr 15;88(4):640-51. doi: 10.1016/j.bcp.2013.12.024. Epub 2014 Jan 4. Biochem Pharmacol. 2014. PMID: 24398425 Free PMC article. Review.
-
Brain PET amyloid and neurodegeneration biomarkers in the context of the 2018 NIA-AA research framework: an individual approach exploring clinical-biomarker mismatches and sociodemographic parameters.Eur J Nucl Med Mol Imaging. 2020 Oct;47(11):2666-2680. doi: 10.1007/s00259-020-04714-0. Epub 2020 Feb 13. Eur J Nucl Med Mol Imaging. 2020. PMID: 32055966
References
-
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed, text revision. Washington DC: American Psychiatric Association; 2000.
-
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. - PubMed
-
- Delacourte A, David JP, Sergeant N, Buee L, Wattez A, Vermersch P, et al. The biochemical pathway of neurofibrillary degeneration in aging and Alzheimer’s disease. Neurology. 1999;52:1158–1165. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical