Survivin expression increases during aging and enhances the resistance of aged human fibroblasts to genotoxic stress
- PMID: 22252435
- PMCID: PMC3636406
- DOI: 10.1007/s11357-011-9378-2
Survivin expression increases during aging and enhances the resistance of aged human fibroblasts to genotoxic stress
Abstract
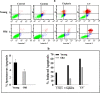
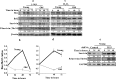
Survivin, an important anti-apoptotic protein, is highly expressed in most cancers, which generally arise in cells of older individuals. We have shown here accumulation of survivin and phospho-survivin in aged normal human skin fibroblasts and mice organs. This age-related accumulation of survivin was due to protein stabilization through association with the molecular chaperone Hsp90 protein, which was also up-regulated during aging. Interestingly, Hsp90 binds preferentially to phospho-survivin, which explains its higher stability. In addition, we provide clear evidence that aged cells exhibit apoptosis resistance when challenged with UV light, cisplatin, γ-rays or H2O2 as compared to their younger counterparts. In response to γ-rays and H2O2, the levels of Bcl-2 and both forms of survivin were up-regulated in old cells, but not in their corresponding young ones. This repression of survivin and phospho-survivin in young cells is p53 dependent. Importantly, survivin inhibition/down-regulation with flavopiridol or specific shRNAs increased the apoptotic response of old fibroblasts to various genotoxic agents, and restored the pro-apoptotic Bax/Bcl2 ratio and the increase in the levels of cleaved caspase-3 and PARP in old cells. These results show the role of survivin in the age-dependent resistance of human fibroblasts, and provide new insights into the molecular mechanisms that underlie the complex relationship between aging, apoptosis, and cancer.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
