Generation of human vascular smooth muscle subtypes provides insight into embryological origin-dependent disease susceptibility
- PMID: 22252507
- PMCID: PMC3272383
- DOI: 10.1038/nbt.2107
Generation of human vascular smooth muscle subtypes provides insight into embryological origin-dependent disease susceptibility
Abstract
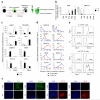
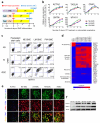
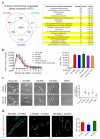
Heterogeneity of embryological origins is a hallmark of vascular smooth muscle cells (SMCs) and may influence the development of vascular disease. Differentiation of human pluripotent stem cells (hPSCs) into developmental origin-specific SMC subtypes remains elusive. Here we describe a chemically defined protocol in which hPSCs were initially induced to form neuroectoderm, lateral plate mesoderm or paraxial mesoderm. These intermediate populations were further differentiated toward SMCs (>80% MYH11(+) and ACTA2(+)), which displayed contractile ability in response to vasoconstrictors and invested perivascular regions in vivo. Derived SMC subtypes recapitulated the unique proliferative and secretory responses to cytokines previously documented in studies using aortic SMCs of distinct origins. Notably, this system predicted increased extracellular matrix degradation by SMCs derived from lateral plate mesoderm, which was confirmed using rat aortic SMCs from corresponding origins. This differentiation approach will have broad applications in modeling origin-dependent disease susceptibility and in developing bioengineered vascular grafts for regenerative medicine.
Figures



References
-
- Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nature medicine. 2000;6:389–395. - PubMed
-
- Majesky MW. Developmental basis of vascular smooth muscle diversity. Arteriosclerosis, thrombosis, and vascular biology. 2007;27:1248–1258. - PubMed
-
- Waldo KL, et al. Secondary heart field contributes myocardium and smooth muscle to the arterial pole of the developing heart. Developmental biology. 2005;281:78–90. - PubMed
-
- Jiang X, Rowitch DH, Soriano P, McMahon AP, Sucov HM. Fate of the mammalian cardiac neural crest. Development. 2000;127:1607–1616. - PubMed
-
- Wasteson P, et al. Developmental origin of smooth muscle cells in the descending aorta in mice. Development. 2008;135:1823–1832. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

