Photoactivatable fluorophores and techniques for biological imaging applications
- PMID: 22252510
- PMCID: PMC3677749
- DOI: 10.1039/c2pp05342j
Photoactivatable fluorophores and techniques for biological imaging applications
Abstract
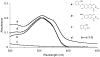
Photoactivatable fluorophores (PAFs) are powerful imaging probes for tracking molecular and cellular dynamics with high spatiotemporal resolution in biological systems. Recent developments in biological microscopy have raised new demands for engineering new PAFs with improved properties, such as high two photon excitation efficiency, reversibility, cellular delivery and targeting. Here we review the history and some of the recent developments in this area, emphasizing our efforts in developing a new class of caged coumarins and related imaging methods for studying dynamic cell-cell communication through gap junction channels, and in extending the application of these caged coumarins to new areas including spatiotemporal control of microRNA activity in vivo.
Figures
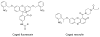
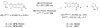




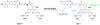


References
-
- Zweig A. Pure and Applied Chemistry. 1973;33:389–410.
-
- Ware BR, Brvenik LJ, Cummings RR, Furukawa RH, Krafft GA. In: Application of fluorescence in the biomedical sciences. Taylor DL, Lanni F, Murphy R, Waggoner A, editors. Alan R. Liss, Inc; New York: 1986. pp. 141–157.
-
- Krafft GA, Cummings RT, Dizio JP, Furukawa RH, Brvenik LJ, Sutton WR, Ware BR. In: Nucleocytoplasmic Transport. Peters R, Trendelenburg M, editors. Springer-Verlag; Berlin: 1986. pp. 35–52.
-
- Krafft GA, Sutton WR, Cummings RT. J Am Chem Soc. 1988;110:301–303.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

