Preventing spontaneous genetic rearrangements in the transgene cassettes of adenovirus vectors
- PMID: 22252512
- PMCID: PMC4981243
- DOI: 10.1002/bit.24342
Preventing spontaneous genetic rearrangements in the transgene cassettes of adenovirus vectors
Abstract
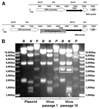
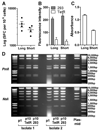
First-generation, E1/E3-deleted adenoviral vectors with diverse transgenes are produced routinely in laboratories worldwide for development of novel prophylactics and therapies for a variety of applications, including candidate vaccines against important infectious diseases, such as HIV/AIDS, tuberculosis, and malaria. Here, we show, for two different transgenes (both encoding malarial antigens) inserted at the E1 locus, that rare viruses containing a transgene-inactivating mutation exhibit a selective growth advantage during propagation in E1-complementing HEK293 cells, such that they rapidly become the major or sole species in the viral population. For one of these transgenes, we demonstrate that viral yield and cytopathic effect are enhanced by repression of transgene expression in the producer cell line, using the tetracycline repressor system. In addition to these transgene-inactivating mutations, one of which occurred during propagation of the pre-viral genomic clone in bacteria, and the other after viral reconstitution in HEK293 cells, we describe two other types of mutation, a small deletion and a gross rearranging duplication, in one of the transgenes studied. These were of uncertain origin, and the effects on transgene expression and viral growth were not fully characterized. We demonstrate that, together with minor protocol modifications, repression of transgene expression in HEK293 cells during viral propagation enables production of a genetically stable chimpanzee adenovirus vector expressing a malarial antigen which had previously been impossible to derive. These results have important implications for basic and pre-clinical studies using adenoviral vectors and for derivation of adenoviral vector products destined for large-scale amplification during biomanufacture.
Copyright © 2011 Wiley Periodicals, Inc.
Conflict of interest statement
Conflict of interest: Okairòs Srl and the University of Oxford hold intellectual property related to adenovirus vaccine vectors.
Figures




Similar articles
-
Construction and characterization of E1- and E3-deleted adenovirus vectors expressing two antigens from two separate expression cassettes.Hum Gene Ther. 2014 Apr;25(4):328-38. doi: 10.1089/hum.2013.216. Epub 2014 Mar 25. Hum Gene Ther. 2014. PMID: 24367921 Free PMC article.
-
A single short stretch of homology between adenoviral vector and packaging cell line can give rise to cytopathic effect-inducing, helper-dependent E1-positive particles.Hum Gene Ther. 2002 May 20;13(8):909-20. doi: 10.1089/10430340252939023. Hum Gene Ther. 2002. PMID: 12031124
-
Concordant activity of transgene expression cassettes inserted into E1, E3 and E4 cloning sites in the adenovirus genome.J Gene Med. 2009 Mar;11(3):197-206. doi: 10.1002/jgm.1289. J Gene Med. 2009. PMID: 19140107 Free PMC article.
-
Common structure of rare replication-deficient E1-positive particles in adenoviral vector batches.J Virol. 2004 Jun;78(12):6200-8. doi: 10.1128/JVI.78.12.6200-6208.2004. J Virol. 2004. PMID: 15163713 Free PMC article.
-
Generation of recombinant adenovirus vector with infectious adenoviral genome released from cosmid-based vector by simple procedure allowing complex manipulation.Biochem Biophys Res Commun. 1998 May 29;246(3):868-72. doi: 10.1006/bbrc.1998.8706. Biochem Biophys Res Commun. 1998. PMID: 9618304
Cited by
-
Development of a Molecular Adjuvant to Enhance Antigen-Specific CD8+ T Cell Responses.Sci Rep. 2018 Oct 9;8(1):15020. doi: 10.1038/s41598-018-33375-1. Sci Rep. 2018. PMID: 30301933 Free PMC article.
-
The utility of Plasmodium berghei as a rodent model for anti-merozoite malaria vaccine assessment.Sci Rep. 2013;3:1706. doi: 10.1038/srep01706. Sci Rep. 2013. PMID: 23609325 Free PMC article.
-
A single dose of ChAdOx1 MERS provides protective immunity in rhesus macaques.Sci Adv. 2020 Jun 10;6(24):eaba8399. doi: 10.1126/sciadv.aba8399. eCollection 2020 Jun. Sci Adv. 2020. PMID: 32577525 Free PMC article.
-
Systemic prime mucosal boost significantly increases protective efficacy of bivalent RSV influenza viral vectored vaccine.NPJ Vaccines. 2024 Jun 26;9(1):118. doi: 10.1038/s41541-024-00912-1. NPJ Vaccines. 2024. PMID: 38926455 Free PMC article.
-
Transgene expression knock-down in recombinant Modified Vaccinia virus Ankara vectors improves genetic stability and sustained transgene maintenance across multiple passages.Front Immunol. 2024 Feb 6;15:1338492. doi: 10.3389/fimmu.2024.1338492. eCollection 2024. Front Immunol. 2024. PMID: 38380318 Free PMC article.
References
-
- Abbink P, Lemckert AA, Ewald BA, Lynch DM, Denholtz M, Smits S, Holterman L, Damen I, Vogels R, Thorner AR, O’Brien KL, et al. Comparative seroprevalence and immunogenicity of six rare serotype recombinant adenovirus vaccine vectors from subgroups B and D. J Virol. 2007;81(9):4654–4663. - PMC - PubMed
-
- Capone S, Reyes-Sandoval A, Naddeo M, Siani L, Ammendola V, Rollier CS, Nicosia A, Colloca S, Cortese R, Folgori A, Hill AV. Immune responses against a liver-stage malaria antigen induced by simian adenoviral vector AdCh63 and MVA prime-boost immunisation in non-human primates. Vaccine. 2010;29(2):256–265. - PubMed
-
- Caravokyri C, Pringle CR, Leppard KN. Human adenovirus type 5 recombinants expressing simian immunodeficiency virus macaque strain gag antigens. J Gen Virol. 1993;74(Pt 12):2819–2824. - PubMed
-
- Draper SJ, Biswas S, Spencer AJ, Remarque EJ, Capone S, Naddeo M, Dicks MD, Faber BW, de Cassan SC, Folgori A, Nicosia A, et al. Enhancing blood-stage malaria subunit vaccine immunogenicity in Rhesus macaques by combining adenovirus, poxvirus, and protein-in-adjuvant vaccines. J Immunol. 2010;185(12):7583–7595. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

