Outcome for adolescent and young adult patients with osteosarcoma: a report from the Children's Oncology Group
- PMID: 22252521
- PMCID: PMC4008337
- DOI: 10.1002/cncr.27414
Outcome for adolescent and young adult patients with osteosarcoma: a report from the Children's Oncology Group
Abstract
Background: There are conflicting data regarding age as a prognostic factor in osteosarcoma. The authors conducted a study evaluating the impact of age on prognosis in children and young adults with osteosarcoma enrolled on North American cooperative group trials.
Methods: Patients with high-grade osteosarcoma of any site enrolled on North American cooperative group trials CCG-7943, POG-9754, INT-0133, and AOST0121 were included in this study. Primary tumor site, age, sex, ethnicity, histologic response, and presence of metastatic disease at diagnosis were evaluated for their impact on overall survival (OS) and event-free survival (EFS).
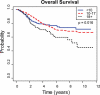
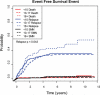
Results: A total of 1054 patients were eligible and had complete data available for the study. Age was not significantly associated with any other presenting covariate analyzed except sex. Age 18 or older was associated with a statistically significant poorer EFS (P = .019) and OS (P = .043). The 10-year EFS and OS in patients <10, 10 to 17, and ≥18 years old were 55%, 55%, 37% and 68%, 60%, 41%, respectively. The poorer EFS in patients ≥18 years old was because of an increased rate of relapse. Presence of metastatic disease at diagnosis, poor histologic response, and pelvic tumor site were also associated with a poorer prognosis. In multivariate analysis, age continued to be associated with poorer EFS (P = .019) and OS (P = .049).
Conclusions: In osteosarcoma, age 18 to 30 years is associated with a statistically significant poorer outcome because of an increased rate of relapse. Poorer outcome in adolescent and young adult patients is not explained by tumor location, histologic response, or metastatic disease at presentation.
Copyright © 2012 American Cancer Society.
Figures



References
-
- Adolescent and Young Adult Oncology Progress Review Group . Closing the gap: research and care imperatives for adolescents and young adults with cancer. NIH Pub. No. 06-6067. National Institutes of Health; Bethesda, MD: 2006. Available at: http://planning. cancer.gov/library/AYAO_PRG_Report_2006_FINAL.pdf.
-
- Bleyer A, Barr R, Hayes-Lattin B, Thomas D, Ellis C, Anderson B. The distinctive biology of cancer in adolescents and young adults. Nat Rev Cancer. 2008;8:288–298. - PubMed
-
- Grier HE, Krailo MD, Tarbell NJ, et al. Addition of ifosfamide and etoposide to standard chemotherapy for Ewing's sarcoma and primitive neuroectodermal tumor of bone. N Engl J Med. 2003;348:694–701. - PubMed
-
- Craft A, Cotterill S, Malcolm A, et al. Ifosfamide-containing chemotherapy in Ewing's sarcoma: the Second United Kingdom Children's Cancer Study Group and the Medical Research Council Ewing's Tumor Study. J Clin Oncol. 1998;16:3628–3633. - PubMed
-
- Sultan I, Qaddoumi I, Yaser S, Rodriguez-Galindo C, Ferrari A. Comparing adult and pediatric rhabdomyosarcoma in the Surveillance, Epidemiology and End Results program, 1973 to 2005: an analysis of 2,600 patients. J Clin Oncol. 2009;27:3391–3397. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

