Mice with a D190N mutation in the gene encoding rhodopsin: a model for human autosomal-dominant retinitis pigmentosa
- PMID: 22252712
- PMCID: PMC3388123
- DOI: 10.2119/molmed.2011.00475
Mice with a D190N mutation in the gene encoding rhodopsin: a model for human autosomal-dominant retinitis pigmentosa
Abstract
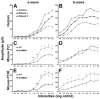
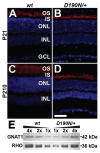
Rhodopsin is the G protein-coupled receptor in charge of initiating signal transduction in rod photoreceptor cells upon the arrival of the photon. D190N (Rho(D190n)), a missense mutation in rhodopsin, causes autosomal-dominant retinitis pigmentosa (adRP) in humans. Affected patients present hyperfluorescent retinal rings and progressive rod photoreceptor degeneration. Studies in humans cannot reveal the molecular processes causing the earliest stages of the condition, thus necessitating the creation of an appropriate animal model. A knock-in mouse model with the D190N mutation was engineered to study the pathogenesis of the disease. Electrophysiological and histological findings in the mouse were similar to those observed in human patients, and the hyperfluorescence pattern was analogous to that seen in humans, confirming that the D190N mouse is an accurate model for the study of adRP.
Figures



References
-
- Berson EL. Retinitis pigmentosa: the Friedenwald Lecture. Invest Ophthalmol Vis Sci. 1993;34:1659–76. - PubMed
-
- Hartong DT, Berson EL, Dryja TP. Retinitis pigmentosa. Lancet. 2006;368:1795–809. - PubMed
-
- Rivolta C, Sharon D, DeAngelis MM, Dryja TP. Retinitis pigmentosa and allied diseases: numerous diseases, genes, and inheritance patterns. Hum Mol Genet. 2002;11:1219–27. - PubMed
-
- Wilson JH, Wensel TG. The nature of dominant mutations of rhodopsin and implications for gene therapy. Mol Neurobiol. 2003;28:149–58. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

