SQ109 targets MmpL3, a membrane transporter of trehalose monomycolate involved in mycolic acid donation to the cell wall core of Mycobacterium tuberculosis
- PMID: 22252828
- PMCID: PMC3318387
- DOI: 10.1128/AAC.05708-11
SQ109 targets MmpL3, a membrane transporter of trehalose monomycolate involved in mycolic acid donation to the cell wall core of Mycobacterium tuberculosis
Abstract
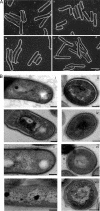
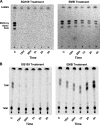
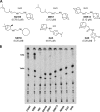
SQ109, a 1,2-diamine related to ethambutol, is currently in clinical trials for the treatment of tuberculosis, but its mode of action remains unclear. Here, we demonstrate that SQ109 disrupts cell wall assembly, as evidenced by macromolecular incorporation assays and ultrastructural analyses. SQ109 interferes with the assembly of mycolic acids into the cell wall core of Mycobacterium tuberculosis, as bacilli exposed to SQ109 show immediate inhibition of trehalose dimycolate (TDM) production and fail to attach mycolates to the cell wall arabinogalactan. These effects were not due to inhibition of mycolate synthesis, since total mycolate levels were unaffected, but instead resulted in the accumulation of trehalose monomycolate (TMM), the precursor of TDM and cell wall mycolates. In vitro assays using purified enzymes showed that this was not due to inhibition of the secreted Ag85 mycolyltransferases. We were unable to achieve spontaneous generation of SQ109-resistant mutants; however, analogs of this compound that resulted in similar shutdown of TDM synthesis with concomitant TMM accumulation were used to spontaneously generate resistant mutants that were also cross-resistant to SQ109. Whole-genome sequencing of these mutants showed that these all had mutations in the essential mmpL3 gene, which encodes a transmembrane transporter. Our results suggest that MmpL3 is the target of SQ109 and that MmpL3 is a transporter of mycobacterial TMM.
Figures






References
-
- Barry CE, III, et al. 1998. Mycolic acids: structure, biosynthesis and physiological functions. Prog. Lipid Res. 37:143–179 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

