Hypoxia-mediated impairment of the mitochondrial respiratory chain inhibits the bactericidal activity of macrophages
- PMID: 22252868
- PMCID: PMC3318416
- DOI: 10.1128/IAI.05972-11
Hypoxia-mediated impairment of the mitochondrial respiratory chain inhibits the bactericidal activity of macrophages
Abstract
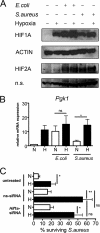
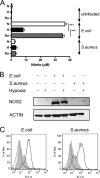
In infected tissues oxygen tensions are low. As innate immune cells have to operate under these conditions, we analyzed the ability of macrophages (Mφ) to kill Escherichia coli or Staphylococcus aureus in a hypoxic microenvironment. Oxygen restriction did not promote intracellular bacterial growth but did impair the bactericidal activity of the host cells against both pathogens. This correlated with a decreased production of reactive oxygen intermediates (ROI) and reactive nitrogen intermediates. Experiments with phagocyte NADPH oxidase (PHOX) and inducible NO synthase (NOS2) double-deficient Mφ revealed that in E. coli- or S. aureus-infected cells the reduced antibacterial activity during hypoxia was either entirely or partially independent of the diminished PHOX and NOS2 activity. Hypoxia impaired the mitochondrial activity of infected Mφ. Inhibition of the mitochondrial respiratory chain activity during normoxia (using rotenone or antimycin A) completely or partially mimicked the defective antibacterial activity observed in hypoxic E. coli- or S. aureus-infected wild-type Mφ, respectively. Accordingly, inhibition of the respiratory chain of S. aureus-infected, normoxic PHOX(-/-) NOS2(-/-) Mφ further raised the bacterial burden of the cells, which reached the level measured in hypoxic PHOX(-/-) NOS2(-/-) Mφ cultures. Our data demonstrate that the reduced killing of S. aureus or E. coli during hypoxia is not simply due to a lack of PHOX and NOS2 activity but partially or completely results from an impaired mitochondrial antibacterial effector function. Since pharmacological inhibition of the respiratory chain raised the generation of ROI but nevertheless phenocopied the effect of hypoxia, ROI can be excluded as the mechanism underlying the antimicrobial activity of mitochondria.
Figures




Similar articles
-
Phenotype of mice and macrophages deficient in both phagocyte oxidase and inducible nitric oxide synthase.Immunity. 1999 Jan;10(1):29-38. doi: 10.1016/s1074-7613(00)80004-7. Immunity. 1999. PMID: 10023768
-
HIF1A and NFAT5 coordinate Na+-boosted antibacterial defense via enhanced autophagy and autolysosomal targeting.Autophagy. 2019 Nov;15(11):1899-1916. doi: 10.1080/15548627.2019.1596483. Epub 2019 Apr 14. Autophagy. 2019. PMID: 30982460 Free PMC article.
-
Low-oxygen tensions found in Salmonella-infected gut tissue boost Salmonella replication in macrophages by impairing antimicrobial activity and augmenting Salmonella virulence.Cell Microbiol. 2015 Dec;17(12):1833-47. doi: 10.1111/cmi.12476. Epub 2015 Jul 16. Cell Microbiol. 2015. PMID: 26104016
-
Mitochondrial generation of free radicals and hypoxic signaling.Trends Endocrinol Metab. 2009 Sep;20(7):332-40. doi: 10.1016/j.tem.2009.04.001. Epub 2009 Sep 3. Trends Endocrinol Metab. 2009. PMID: 19733481 Review.
-
Oxygen-dependent anti-Salmonella activity of macrophages.Trends Microbiol. 2001 Jan;9(1):29-33. doi: 10.1016/s0966-842x(00)01897-7. Trends Microbiol. 2001. PMID: 11166240 Review.
Cited by
-
Macrophage mitochondrial and stress response to ingestion of Cryptococcus neoformans.J Immunol. 2015 Mar 1;194(5):2345-57. doi: 10.4049/jimmunol.1402350. Epub 2015 Feb 2. J Immunol. 2015. PMID: 25646306 Free PMC article.
-
Macrophage Polarization and Reprogramming in Acute Inflammation: A Redox Perspective.Antioxidants (Basel). 2022 Jul 19;11(7):1394. doi: 10.3390/antiox11071394. Antioxidants (Basel). 2022. PMID: 35883885 Free PMC article. Review.
-
Parkinsonian Neurotoxins Impair the Pro-inflammatory Response of Glial Cells.Front Mol Neurosci. 2019 Jan 10;11:479. doi: 10.3389/fnmol.2018.00479. eCollection 2018. Front Mol Neurosci. 2019. PMID: 30686998 Free PMC article.
-
Indoleamine 2,3-dioxygenase expression regulates the survival and proliferation of Fusobacterium nucleatum in THP-1-derived macrophages.Cell Death Dis. 2018 Mar 2;9(3):355. doi: 10.1038/s41419-018-0389-0. Cell Death Dis. 2018. PMID: 29500439 Free PMC article.
-
Hypoxia in Leishmania major skin lesions impairs the NO-dependent leishmanicidal activity of macrophages.J Invest Dermatol. 2014 Sep;134(9):2339-2346. doi: 10.1038/jid.2014.121. Epub 2014 Feb 28. J Invest Dermatol. 2014. PMID: 24583949
References
-
- Ackermann M, et al. 2008. Self-destructive cooperation mediated by phenotypic noise. Nature 454:987–990 - PubMed
-
- Albina JE, Henry WL, Jr, Mastrofrancesco B, Martin BA, Reichner JS. 1995. Macrophage activation by culture in an anoxic environment. J. Immunol. 155:4391–4396 - PubMed
-
- Anand RJ, et al. 2007. Hypoxia causes an increase in phagocytosis by macrophages in a HIF-1α-dependent manner. J. Leukoc. Biol. 82:1257–1265 - PubMed
-
- Arsenijevic D, et al. 2000. Disruption of the uncoupling protein-2 gene in mice reveals a role in immunity and reactive oxygen species production. Nat. Genet. 26:435–439 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

