A hybrid multistage protein vaccine induces protective immunity against murine malaria
- PMID: 22252877
- PMCID: PMC3318412
- DOI: 10.1128/IAI.05980-11
A hybrid multistage protein vaccine induces protective immunity against murine malaria
Abstract
We have previously reported the design and expression of chimeric recombinant proteins as an effective platform to deliver malaria vaccines. The erythrocytic and exoerythrocytic protein chimeras described included autologous T helper epitopes genetically linked to defined B cell epitopes. Proof-of-principle studies using vaccine constructs based on the Plasmodium yoelii circumsporozoite protein (CSP) and P. yoelii merozoite surface protein-1 (MSP-1) showed encouraging results when tested individually in this mouse malaria model. To evaluate the potential synergistic or additive effect of combining these chimeric antigens, we constructed a synthetic gene encoding a hybrid protein that combined both polypeptides in a single immunogen. The multistage vaccine was expressed in soluble form in Escherichia coli at high yield. Here we report that the multistage protein induced robust immune responses to individual components, with no evidence of vaccine interference. Passive immunization using purified IgG from rabbits immunized with the hybrid protein conferred more robust protection against the experimental challenge with P. yoelii sporozoites than passive immunization with purified IgG from rabbits immunized with the individual proteins. High antibody titers and high frequencies of CD4(+)- and CD8(+)-specific cytokine-secreting T cells were elicited by vaccination. T cells were multifunctional and able to simultaneously produce interleukin-2 (IL-2), gamma interferon (IFN-γ), and tumor necrosis factor alpha (TNF-α). The mechanism of vaccine-induced protection involved neutralizing antibodies and effector CD4(+) T cells and resulted in the control of hyperparasitemia and protection against malarial anemia. These data support our strategy of using an array of autologous T helper epitopes to maximize the response to multistage malaria vaccines.
Figures
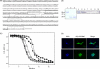
 ), 3D11 (▽), or anti-6×His tag (●). Data are presented as the geometric mean OD obtained at different concentrations of the corresponding monoclonal antibodies (10 μg/ml to 0.00047 ng/ml) or the reciprocal of the serum dilution obtained from mice immunized with P. yoelii PyLPC/RMC, PyLPC, or PyRMC (dilutions 1:1,000 to 1:209,715,200). Numbers on the x axis indicate the dilutions tested (dilution number 1 was 10 μg/ml for monoclonal antibodies or 1:1,000 for polyclonal antibodies). Antigen specificity was confirmed using preimmune serum samples as a control (data not shown). (D) Immunofluorescence images of P. yoelii parasites (sporozoites and schizonts) obtained by incubation with anti-PyLPC/RMC antibodies showing the characteristic staining patterns of CSP and MSP-1.
), 3D11 (▽), or anti-6×His tag (●). Data are presented as the geometric mean OD obtained at different concentrations of the corresponding monoclonal antibodies (10 μg/ml to 0.00047 ng/ml) or the reciprocal of the serum dilution obtained from mice immunized with P. yoelii PyLPC/RMC, PyLPC, or PyRMC (dilutions 1:1,000 to 1:209,715,200). Numbers on the x axis indicate the dilutions tested (dilution number 1 was 10 μg/ml for monoclonal antibodies or 1:1,000 for polyclonal antibodies). Antigen specificity was confirmed using preimmune serum samples as a control (data not shown). (D) Immunofluorescence images of P. yoelii parasites (sporozoites and schizonts) obtained by incubation with anti-PyLPC/RMC antibodies showing the characteristic staining patterns of CSP and MSP-1.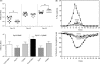
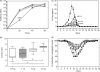

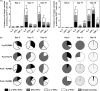

References
-
- Bentley GA. 2006. Functional and immunological insights from the three-dimensional structures of Plasmodium surface proteins. Curr. Opin. Microbiol. 9:395–400 - PubMed
-
- Caro-Aguilar I, Lapp S, Pohl J, Galinski MR, Moreno A. 2005. Chimeric epitopes delivered by polymeric synthetic linear peptides induce protective immunity to malaria. Microbes Infect. 7:1324–1337 - PubMed
-
- Casares S, Brumeanu T-D, Richie TL. 2010. The RTS,S malaria vaccine. Vaccine 28:4880–4894 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

