Resumption of high-dose methotrexate after acute kidney injury and glucarpidase use in pediatric oncology patients
- PMID: 22252903
- PMCID: PMC3713608
- DOI: 10.1002/cncr.27378
Resumption of high-dose methotrexate after acute kidney injury and glucarpidase use in pediatric oncology patients
Abstract
Background: High-dose methotrexate (HDMTX)-induced acute kidney injury is a rare but life-threatening complication. The methotrexate rescue agent glucarpidase rapidly hydrolyzes methotrexate to inactive metabolites. The authors retrospectively reviewed glucarpidase use in pediatric cancer patients at their institution and evaluated whether subsequent resumption of HDMTX was tolerated.
Methods: Clinical data and outcomes of all patients who received glucarpidase after HDMTX administration were reviewed.
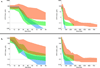
Results: Of 1141 patients who received 4909 courses of HDMTX, 20 patients (1.8% of patients, 0.4% of courses) received 22 doses of glucarpidase. The median glucarpidase dose was 51.6 U/kg (range, 13-65.6 U/kg). At the time of administration, the median plasma methotrexate concentration was 29.1 μM (range, 1.3-590.6 μM). Thirteen of the 20 patients received a total of 39 courses of HDMTX therapy after glucarpidase. The median time to complete methotrexate excretion was 355 hours (range, 244-763 hours) for the HDMTX course during which glucarpidase was administered, 90 hours (range, 66-268 hours) for the next HDMTX course, and 72 hours (range, 42-116 hours) for subsequent courses. The median peak serum creatinine level during these HDMTX courses was 2.2 mg/dL (range, 0.8-9.6 mg/dL), 0.8 mg/dL (range, 0.4-1.6 mg/dL), and 0.6 mg/dL (range, 0.4-0.9 mg/dL), respectively. One patient experienced nephrotoxicity upon rechallenge with HDMTX. Renal function eventually returned to baseline in all patients, and no patient died as a result of methotrexate toxicity.
Conclusions: The current results indicated that it is possible to safely resume HDMTX therapy after glucarpidase treatment for HDMTX-induced acute kidney injury.
Copyright © 2012 American Cancer Society.
Conflict of interest statement
Financial Disclosures: The authors declare no financial conflicts of interest.
Figures

References
-
- Treon SP, Chabner BA. Concepts in use of high-dose methotrexate therapy. Clin Chem. 1996;42:1322–1329. - PubMed
-
- Widemann BC, Adamson PC. Understanding and managing methotrexate nephrotoxicity. Oncologist. 2006;11:694–703. - PubMed
-
- Patterson DM, Lee SM. Glucarpidase following high-dose methotrexate: update on development. Expert Opin Biol Ther. 2010;10:105–111. - PubMed
-
- Schwartz S, Borner K, Muller K, et al. Glucarpidase (carboxypeptidase g2) intervention in adult and elderly cancer patients with renal dysfunction and delayed methotrexate elimination after high-dose methotrexate therapy. Oncologist. 2007;12:1299–1308. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

