Rab25 increases cellular ATP and glycogen stores protecting cancer cells from bioenergetic stress
- PMID: 22253197
- PMCID: PMC3306554
- DOI: 10.1002/emmm.201100193
Rab25 increases cellular ATP and glycogen stores protecting cancer cells from bioenergetic stress
Abstract
Cancer cells are metabolically stressed during tumour progression due to limited tumour vascularity and resultant nutrient, growth factor and oxygen deficiency that can induce cell death and inhibit tumour growth. We demonstrate that Rab25, a small GTPase involved in endosomal recycling, that is genomically amplified in multiple tumour lineages, is a key regulator of cellular bioenergetics and autophagy. RAB25 enhanced survival during nutrient stress by preventing apoptosis and autophagy via binding and activating AKT leading to increased glucose uptake and improved cellular bioenergetics. Unexpectedly, Rab25 induced the accumulation of glycogen in epithelial cancer cells, a process not previously identified. Strikingly, an increase in basal ATP levels combined with AKT-dependent increases in glucose uptake and glycogen storage allowed maintenance of ATP levels during bioenergetic stress. The clinical relevance of these findings was validated by the ability of a Rab25-dependent expression profile enriched for bioenergetics targets to identify patients with a poor prognosis. Thus, Rab25 is an unexpected regulator of cellular bioenergetics implicated as a useful biomarker and potential therapeutic target.
Copyright © 2012 EMBO Molecular Medicine.
Figures
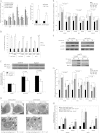

Expression of Rab25 increases endogenous ATP level in ovarian cancer cells. *p < 0.001 Rab25 versus pcDNA. The mean cellular concentration of ATP per mg of protein (based on the standard curve generated using a known amount of ATP) is 2.36−10 moles/mg for IOSE29ht pcDNA cells, 6.21−10 moles/mg for IOSE29ht Rab25 cells, 8.14−11 moles/mg for IOSE80ht pcDNA cells, 1.05−10 moles/mg for IOSE80ht Rab25 cells, 2.85−10 moles/mg for A2780 pcDNA cells, 3.81−10 moles/mg for A2780 Rab25 cells, 7.23−10 moles/mg for HEY pcDNA cells and 9.82−10 moles/mg for HEY Rab25 cells.
Rab25 expression increases ATP synthesis and delays fall in total cellular ATP levels after glucose and serum withdrawal, p < 0.001 Rab25 versus control pcDNA.
Down-regulation of Rab25 expression decreases ability to maintain ATP after glucose and serum withdrawal (a) p < 0.05 SF-Glu versus FBS and (b) p < 0.05 versus control.
Expression of Rab25 increases total cellular glycogen content while down-regulation of Rab25 expression decreases total cellular glycogen content (µg of glycogen/mg protein). Cells were cultured in complete media for 24 h before glycogen measurement. (a) p < 0.01 Rab25 versus pcDNA, (b) p < 0.05 Rab25 RNAi versus NT RNAi and (c) p < 0.05 shRAb25 versus shRNA control.
Decrease in cellular glycogen content after glucose and serum withdrawal *p < 0.001 Rab25 versus pcDNA.
Effect of GPi (Bay U6571) and oligomycin on ATP production. A2780 pcDNA transfected and Rab25 expressing cells were pretreated with vehicle (control), 30 µM of Bay U6571 (GPi) or 25 µg/ml of oligomycin for 2 h. Cells were then cultured in serum- and glucose free RPMI 1640 in the presence of absence of Bay U6571 or oligomycin for the indicated times. (a) p < 0.01 oligomycin time 0 versus control time 0 and (b) p < 0.01 versus time 0 at each treatment group.
Effect of Bay U6571 on cellular glycogen (left panel) and ATP (right panel) levels in HEY cells. (a) p < 0.01 versus pcDNA control time 0 at 5% FBS and (b) p < 0.01 GPi versus control at each corresponding treatment.
Down regulation of Rab25 expression and inhibition of glycogen breakdown by GPi administration increases 2DG induced cell death *p < 0.05 versus shRNA control.

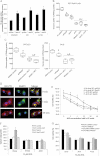

Ovarian cancer HEY cells (upper panel) were co-transfected with AKT-IFPN and Rab25-IFPC or with AKT-IFPC and Rab25-IFPN. Cells become fluorescent due to an interaction between AKT and Rab25 bringing the two halves of the fluorescent protein into proximity creating a stable complex and restoring fluorescence. Hela cells stably expressing AKT-IFPN (lower panel) were transfected with Rab25-IFPC or PDK-IFPC as control. A brightfield image corresponding to the fluorescent image is shown and transfected cells indicated by an arrow.
Detection of Rab25 and AKT interaction by immunoprecipitation. Ovarian cells expressing HA-tagged Rab25 were IP with anti-HA antibody. The resultant complex was separated by gel electrophoresis and detected by WB using anti-AKT antibody.
Generation of Rab25 deletion mutants. Expression of the deletion mutants was detected by immunofluorescence staining (upper panel) and WB (middle panel) using anti-GFP antibody which interacts with YFP. Diagrammatic representation of the structure of the mutants is shown in the lower panel.
Rab25 deletion mutants do not alter cellular glycogen and ATP levels. Ovarian cancer cell lysates were collected 24 h post transfection. (a) p < 0.05 versus empty vector transfected cells.

Similar articles
-
Rab25 in cancer: a brief update.Biochem Soc Trans. 2012 Dec 1;40(6):1404-8. doi: 10.1042/BST20120249. Biochem Soc Trans. 2012. PMID: 23176489 Free PMC article. Review.
-
Knockdown of RAB25 promotes autophagy and inhibits cell growth in ovarian cancer cells.Mol Med Rep. 2012 Nov;6(5):1006-12. doi: 10.3892/mmr.2012.1052. Epub 2012 Aug 28. Mol Med Rep. 2012. PMID: 22940905
-
Structural and functional analysis of FIP2 binding to the endosome-localised Rab25 GTPase.Biochim Biophys Acta. 2013 Dec;1834(12):2679-90. doi: 10.1016/j.bbapap.2013.09.005. Epub 2013 Sep 19. Biochim Biophys Acta. 2013. PMID: 24056041
-
Rab25 and RCP in cancer progression.Arch Pharm Res. 2019 Feb;42(2):101-112. doi: 10.1007/s12272-019-01129-w. Epub 2019 Feb 14. Arch Pharm Res. 2019. PMID: 30761451 Review.
-
Overexpression of Rab25 contributes to metastasis of bladder cancer through induction of epithelial-mesenchymal transition and activation of Akt/GSK-3β/Snail signaling.Carcinogenesis. 2013 Oct;34(10):2401-8. doi: 10.1093/carcin/bgt187. Epub 2013 May 30. Carcinogenesis. 2013. PMID: 23722651
Cited by
-
Rab25 promotes erlotinib resistance by activating the β1 integrin/AKT/β-catenin pathway in NSCLC.Cell Prolif. 2019 May;52(3):e12592. doi: 10.1111/cpr.12592. Epub 2019 Mar 7. Cell Prolif. 2019. PMID: 30848009 Free PMC article.
-
Glucose Metabolism in Cancer.Adv Exp Med Biol. 2018;1063:3-12. doi: 10.1007/978-3-319-77736-8_1. Adv Exp Med Biol. 2018. PMID: 29946772 Review.
-
Expression analysis of the estrogen receptor target genes in renal cell carcinoma.Mol Med Rep. 2015 Jan;11(1):75-82. doi: 10.3892/mmr.2014.2766. Epub 2014 Oct 24. Mol Med Rep. 2015. PMID: 25351113 Free PMC article.
-
Rab25 Small GTPase Mediates Secretion of Tumor Necrosis Factor Receptor Superfamily Member 11b (osteoprotegerin) Protecting Cancer Cells from Effects of TRAIL.J Genet Syndr Gene Ther. 2013;4:1000153. doi: 10.4172/2157-7412.1000153. J Genet Syndr Gene Ther. 2013. PMID: 25520884 Free PMC article.
-
Rab40b upregulation correlates with the prognosis of gastric cancer by promoting migration, invasion, and metastasis.Med Oncol. 2015 Apr;32(4):126. doi: 10.1007/s12032-015-0562-6. Epub 2015 Mar 20. Med Oncol. 2015. PMID: 25790780
References
-
- Caswell PT, Spence HJ, Parsons M, White DP, Clark K, Cheng KW, Mills GB, Humphries MJ, Messent AJ, Anderson KI, et al. Rab25 associates with alpha5beta1 integrin to promote invasive migration in 3D microenvironments. Dev Cell. 2007;13:496–510. - PubMed
-
- Cheng KW, Lahad JP, Kuo WL, Lapuk A, Yamada K, Auersperg N, Liu J, Smith-McCune K, Lu KH, Fishman D, et al. The RAB25 small GTPase determines aggressiveness of ovarian and breast cancers. Nat Med. 2004;10:1251–1256. - PubMed
-
- Cheng KW, Lu Y, Mills GB. Assay of Rab25 function in ovarian and breast cancers. Method Enzymol. 2005;402:202–215. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

