Targeting myocardial substrate metabolism in heart failure: potential for new therapies
- PMID: 22253453
- PMCID: PMC3260022
- DOI: 10.1093/eurjhf/hfr173
Targeting myocardial substrate metabolism in heart failure: potential for new therapies
Abstract
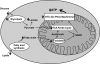
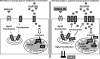
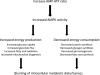
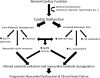
The incidence and prevalence of heart failure have increased significantly over the past few decades. Available data suggest that patients with heart failure independent of the aetiology have viable but dysfunctional myocardium that is potentially salvageable. Although a great deal of research effort has focused on characterizing the molecular basis of heart failure, cardiac metabolism in this disorder remains an understudied discipline. It is known that many aspects of cardiomyocyte energetics are altered in heart failure. These include a shift from fatty acid to glucose as a preferred substrate and a decline in the levels of ATP. Despite these demonstrated changes, there are currently no approved drugs that target metabolic enzymes or proteins in heart failure. This is partly due to our limited knowledge of the mechanisms and pathways that regulate cardiac metabolism. Better characterization of these pathways may potentially lead to new therapies for heart failure. Targeting myocardial energetics in the viable and potentially salvageable tissue may be particularly effective in the treatment of heart failure. Here, we will review metabolic changes that occur in fatty acid and glucose metabolism and AMP-activated kinase in heart failure. We propose that cardiac energetics should be considered as a potential target for therapy in heart failure and more research should be done in this area.
Figures
References
-
- Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics—2010 update: a report from the American Heart Association. Circulation. 2010;121:e46–e215. - PubMed
-
- Baldasseroni S, Opasich C, Gorini M, Lucci D, Marchionni N, Marini M, Campana C, Perini G, Deorsola A, Masotti G, Tavazzi L, Maggioni AP. Left bundle-branch block is associated with increased 1-year sudden and total mortality rate in 5517 outpatients with congestive heart failure: a report from the Italian network on congestive heart failure. Am Heart J. 2002;143:398–405. - PubMed
-
- Lowes BD, Gilbert EM, Abraham WT, Minobe WA, Larrabee P, Ferguson D, Wolfel EE, Lindenfeld J, Tsvetkova T, Robertson AD, Quaife RA, Bristow MR. Myocardial gene expression in dilated cardiomyopathy treated with beta-blocking agents. N Engl J Med. 2002;346:1357–1365. - PubMed
-
- Cleland JG, Pennell DJ, Ray SG, Coats AJ, Macfarlane PW, Murray GD, Mule JD, Vered Z, Lahiri A. Myocardial viability as a determinant of the ejection fraction response to carvedilol in patients with heart failure (CHRISTMAS trial): randomised controlled trial. Lancet. 2003;362:14–21. - PubMed
-
- Taegtmeyer H. Cardiac metabolism as a target for the treatment of heart failure. Circulation. 2004;110:894–896. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

