What will it take to eliminate pediatric HIV? Reaching WHO target rates of mother-to-child HIV transmission in Zimbabwe: a model-based analysis
- PMID: 22253579
- PMCID: PMC3254654
- DOI: 10.1371/journal.pmed.1001156
What will it take to eliminate pediatric HIV? Reaching WHO target rates of mother-to-child HIV transmission in Zimbabwe: a model-based analysis
Abstract
Background: The World Health Organization (WHO) has called for the "virtual elimination" of pediatric HIV: a mother-to-child HIV transmission (MTCT) risk of less than 5%. We investigated uptake of prevention of MTCT (PMTCT) services, infant feeding recommendations, and specific drug regimens necessary to achieve this goal in Zimbabwe.
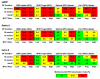
Methods and findings: We used a computer model to simulate a cohort of HIV-infected, pregnant/breastfeeding women (mean age, 24 y; mean CD4, 451/µl; breastfeeding duration, 12 mo). Three PMTCT regimens were evaluated: (1) single-dose nevirapine (sdNVP), (2) WHO 2010 guidelines' "Option A" (zidovudine in pregnancy, infant nevirapine throughout breastfeeding for women without advanced disease, lifelong combination antiretroviral therapy for women with advanced disease), and (3) WHO "Option B" (pregnancy/breastfeeding-limited combination antiretroviral drug regimens without advanced disease; lifelong antiretroviral therapy with advanced disease). We examined four levels of PMTCT uptake (proportion of pregnant women accessing and adhering to PMTCT services): reported rates in 2008 and 2009 (36% and 56%, respectively) and target goals in 2008 and 2009 (80% and 95%, respectively). The primary model outcome was MTCT risk at weaning. The 2008 sdNVP-based National PMTCT Program led to a projected 12-mo MTCT risk of 20.3%. Improved uptake in 2009 reduced projected risk to 18.0%. If sdNVP were replaced by more effective regimens, with 2009 (56%) uptake, estimated MTCT risk would be 14.4% (Option A) or 13.4% (Option B). Even with 95% uptake of Option A or B, projected transmission risks (6.1%-7.7%) would exceed the WHO goal of less than 5%. Only if the lowest published transmission risks were used for each drug regimen, or breastfeeding duration were shortened, would MTCT risks at 95% uptake fall below 5%.
Conclusions: Implementation of the WHO PMTCT guidelines must be accompanied by efforts to improve access to PMTCT services, retain women in care, and support medication adherence throughout pregnancy and breastfeeding, to approach the "virtual elimination" of pediatric HIV in Zimbabwe. Please see later in the article for the Editors' Summary.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Tubiana R, Le Chenadec J, Rouzioux C, Mandelbrot L, Hamrene K, et al. Factors associated with mother-to-child transmission of HIV-1 despite a maternal viral load<500 copies/ml at delivery: a case-control study nested in the French perinatal cohort (EPF-ANRS CO1). Clin Infect Dis. 2010;50:585–596. - PubMed
-
- United States Centers for Disease Control and Prevention. Diagnoses of HIV infection by age. 2010. Available: http://www.cdc.gov/hiv/topics/surveillance/basic.htm#hivaidsage. Accessed 20 July 2011.
-
- World Health Organization. Guidelines on HIV and infant feeding 2010: principles and recommendations for infant feeding in the context of HIV and a summary of evidence. 2010. Available: http://www.who.int/child_adolescent_health/documents/9789241599535/en/in.... Accessed 20 July 2011. - PubMed
-
- Mofenson L, Taha T, Li Q, Kumwenda J, Kafulafula G, et al. Infant extended antiretroviral (ARV) prophylaxis is effective in preventing postnatal mother-to-child HIV transmission (MTCT) at all maternal CD4 counts [abstract]. 5th IAS Conference on HIV Pathogenesis; Treatment and Prevention; 19–22 July 2009; Cape Town, South Africa. 2009. Available: http://www.ias2009.org/pag/Abstracts.aspx?AID=1251. Accessed 20 July 2011.
-
- Vyankandondera J, Luchters S, Hassink E. Reducing risk of HIV-1 transmission from mother to infant through breastfeeding using antiretroviral prophylaxis in infants (SIMBA-study) [abstract]. 2003. 2nd IAS Conference on HIV Pathogenesis and Treatment; 13–16 July 2003; Paris, France.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

