Comparative effectiveness research: an empirical study of trials registered in ClinicalTrials.gov
- PMID: 22253698
- PMCID: PMC3253780
- DOI: 10.1371/journal.pone.0028820
Comparative effectiveness research: an empirical study of trials registered in ClinicalTrials.gov
Abstract
Background: The $1.1 billion investment in comparative effectiveness research will reshape the evidence-base supporting decisions about treatment effectiveness, safety, and cost. Defining the current prevalence and characteristics of comparative effectiveness (CE) research will enable future assessments of the impact of this program.
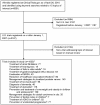
Methods: We conducted an observational study of clinical trials addressing priority research topics defined by the Institute of Medicine and conducted in the US between 2007 and 2010. Trials were identified in ClinicalTrials.gov. Main outcome measures were the prevalence of comparative effectiveness research, nature of comparators selected, funding sources, and impact of these factors on results.
Results: 231 (22.3%; 95% CI 19.8%-24.9%) studies were CE studies and 804 (77.7%; 95% CI, 75.1%-80.2%) were non-CE studies, with 379 (36.6%; 95% CI, 33.7%-39.6%) employing a placebo control and 425 (41.1%; 95% CI, 38.1%-44.1%) no control. The most common treatments examined in CE studies were drug interventions (37.2%), behavioral interventions (28.6%), and procedures (15.6%). Study findings were favorable for the experimental treatment in 34.8% of CE studies and greater than twice as many (78.6%) non-CE studies (P<0.001). CE studies were more likely to receive government funding (P = 0.003) and less likely to receive industry funding (P = 0.01), with 71.8% of CE studies primarily funded by a noncommercial source. The types of interventions studied differed based on funding source, with 95.4% of industry trials studying a drug or device. In addition, industry-funded CE studies were associated with the fewest pediatric subjects (P<0.001), the largest anticipated sample size (P<0.001), and the shortest study duration (P<0.001).
Conclusions: In this sample of studies examining high priority areas for CE research, less than a quarter are CE studies and the majority is supported by government and nonprofits. The low prevalence of CE research exists across CE studies with a broad array of interventions and characteristics.
Conflict of interest statement
Figures
References
-
- Federal Coordinating Council for Comparative Effectiveness Research . Washington, DC: Department of Health and Human Services; 2009. Report to the President and the Congress.
-
- Rawlins MD. NICE work–providing guidance to the British National Health Service. N Engl J Med. 2004;351:1383–1385. - PubMed
-
- Steinbrook R. Saying no isn't NICE - the travails of Britain's National Institute for Health and Clinical Excellence. N Engl J Med. 2008;359:1977–1981. - PubMed
-
- Clement FM, Harris A, Li JJ, Yong K, Lee KM, et al. Using effectiveness and cost-effectiveness to make drug coverage decisions: a comparison of Britain, Australia, and Canada. JAMA. 2009;302:1437–1443. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical