NMR studies on structure and dynamics of the monomeric derivative of BS-RNase: new insights for 3D domain swapping
- PMID: 22253705
- PMCID: PMC3257227
- DOI: 10.1371/journal.pone.0029076
NMR studies on structure and dynamics of the monomeric derivative of BS-RNase: new insights for 3D domain swapping
Erratum in
- PLoS One. 2012;7(2). doi: 10.1371/annotation/b2acf16f-53da-4a8f-a46f-573b7f6f08b7
Abstract
Three-dimensional domain swapping is a common phenomenon in pancreatic-like ribonucleases. In the aggregated state, these proteins acquire new biological functions, including selective cytotoxicity against tumour cells. RNase A is able to dislocate both N- and C-termini, but usually this process requires denaturing conditions. In contrast, bovine seminal ribonuclease (BS-RNase), which is a homo-dimeric protein sharing 80% of sequence identity with RNase A, occurs natively as a mixture of swapped and unswapped isoforms. The presence of two disulfides bridging the subunits, indeed, ensures a dimeric structure also to the unswapped molecule. In vitro, the two BS-RNase isoforms interconvert under physiological conditions. Since the tendency to swap is often related to the instability of the monomeric proteins, in these paper we have analysed in detail the stability in solution of the monomeric derivative of BS-RNase (mBS) by a combination of NMR studies and Molecular Dynamics Simulations. The refinement of NMR structure and relaxation data indicate a close similarity with RNase A, without any evidence of aggregation or partial opening. The high compactness of mBS structure is confirmed also by H/D exchange, urea denaturation, and TEMPOL mapping of the protein surface. The present extensive structural and dynamic investigation of (monomeric) mBS did not show any experimental evidence that could explain the known differences in swapping between BS-RNase and RNase A. Hence, we conclude that the swapping in BS-RNase must be influenced by the distinct features of the dimers, suggesting a prominent role for the interchain disulfide bridges.
Conflict of interest statement
Figures
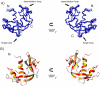



Similar articles
-
The swapping of terminal arms in ribonucleases: comparison of the solution structure of monomeric bovine seminal and pancreatic ribonucleases.Biochemistry. 2003 Jul 29;42(29):8704-11. doi: 10.1021/bi0342517. Biochemistry. 2003. PMID: 12873130
-
The multiple forms of bovine seminal ribonuclease: structure and stability of a C-terminal swapped dimer.FEBS Lett. 2013 Nov 29;587(23):3755-62. doi: 10.1016/j.febslet.2013.10.003. Epub 2013 Oct 15. FEBS Lett. 2013. PMID: 24140346
-
A new mutant of bovine seminal ribonuclease with a reversed swapping propensity.Biochemistry. 2007 Feb 27;46(8):2227-32. doi: 10.1021/bi0613630. Epub 2007 Feb 1. Biochemistry. 2007. PMID: 17269658
-
Structural and functional relationships of natural and artificial dimeric bovine ribonucleases: new scaffolds for potential antitumor drugs.FEBS Lett. 2013 Nov 15;587(22):3601-8. doi: 10.1016/j.febslet.2013.09.038. Epub 2013 Oct 7. FEBS Lett. 2013. PMID: 24113657 Review.
-
3D domain swapping: as domains continue to swap.Protein Sci. 2002 Jun;11(6):1285-99. doi: 10.1110/ps.0201402. Protein Sci. 2002. PMID: 12021428 Free PMC article. Review.
Cited by
-
Mechanism of 3D domain swapping in bovine seminal ribonuclease.FEBS J. 2014 Feb;281(3):842-50. doi: 10.1111/febs.12651. FEBS J. 2014. PMID: 24616921 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

