Lack of effective anti-apoptotic activities restricts growth of Parachlamydiaceae in insect cells
- PMID: 22253735
- PMCID: PMC3253803
- DOI: 10.1371/journal.pone.0029565
Lack of effective anti-apoptotic activities restricts growth of Parachlamydiaceae in insect cells
Abstract
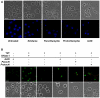
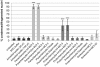
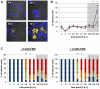
The fundamental role of programmed cell death in host defense is highlighted by the multitude of anti-apoptotic strategies evolved by various microbes, including the well-known obligate intracellular bacterial pathogens Chlamydia trachomatis and Chlamydia (Chlamydophila) pneumoniae. As inhibition of apoptosis is assumed to be essential for a successful infection of humans by these chlamydiae, we analyzed the anti-apoptotic capacity of close relatives that occur as symbionts of amoebae and might represent emerging pathogens. While Simkania negevensis was able to efficiently replicate within insect cells, which served as model for metazoan-derived host cells, the Parachlamydiaceae (Parachlamydia acanthamoebae and Protochlamydia amoebophila) displayed limited intracellular growth, yet these bacteria induced typical features of apoptotic cell death, including formation of apoptotic bodies, nuclear condensation, internucleosomal DNA fragmentation, and effector caspase activity. Induction of apoptosis was dependent on bacterial activity, but not bacterial de novo protein synthesis, and was detectable already at very early stages of infection. Experimental inhibition of host cell death greatly enhanced parachlamydial replication, suggesting that lack of potent anti-apoptotic activities in Parachlamydiaceae may represent an important factor compromising their ability to successfully infect non-protozoan hosts. These findings highlight the importance of the evolution of anti-apoptotic traits for the success of chlamydiae as pathogens of humans and animals.
Conflict of interest statement
Figures




Similar articles
-
Apoptosis Functions in Defense against Infection of Mammalian Cells with Environmental Chlamydiae.Infect Immun. 2020 May 20;88(6):e00851-19. doi: 10.1128/IAI.00851-19. Print 2020 May 20. Infect Immun. 2020. PMID: 32179584 Free PMC article.
-
Parachlamydiaceae: potential emerging pathogens.Emerg Infect Dis. 2002 Jun;8(6):625-30. doi: 10.3201/eid0806.010210. Emerg Infect Dis. 2002. PMID: 12023921 Free PMC article. Review.
-
Antibiotic susceptibility of Neochlamydia hartmanellae and Parachlamydia acanthamoebae in amoebae.Microbes Infect. 2015 Nov-Dec;17(11-12):761-5. doi: 10.1016/j.micinf.2015.08.002. Epub 2015 Aug 14. Microbes Infect. 2015. PMID: 26279002
-
Amoebal host range, host-free survival and disinfection susceptibility of environmental Chlamydiae as compared to Chlamydia trachomatis.FEMS Immunol Med Microbiol. 2012 Apr;64(3):364-73. doi: 10.1111/j.1574-695X.2011.00919.x. Epub 2012 Jan 3. FEMS Immunol Med Microbiol. 2012. PMID: 22141597
-
[Environmental Chlamydiae with medical significance].Dtsch Med Wochenschr. 2011 Oct;136(41):2100-5. doi: 10.1055/s-0031-1292020. Epub 2011 Oct 4. Dtsch Med Wochenschr. 2011. PMID: 21971886 Review. German.
Cited by
-
Host cell death during infection with Chlamydia: a double-edged sword.FEMS Microbiol Rev. 2021 Jan 8;45(1):fuaa043. doi: 10.1093/femsre/fuaa043. FEMS Microbiol Rev. 2021. PMID: 32897321 Free PMC article. Review.
-
Protochlamydia induces apoptosis of human HEp-2 cells through mitochondrial dysfunction mediated by chlamydial protease-like activity factor.PLoS One. 2013;8(2):e56005. doi: 10.1371/journal.pone.0056005. Epub 2013 Feb 11. PLoS One. 2013. PMID: 23409113 Free PMC article.
-
Metabolic features of Protochlamydia amoebophila elementary bodies--a link between activity and infectivity in Chlamydiae.PLoS Pathog. 2013;9(8):e1003553. doi: 10.1371/journal.ppat.1003553. Epub 2013 Aug 8. PLoS Pathog. 2013. PMID: 23950718 Free PMC article.
-
Apoptosis Functions in Defense against Infection of Mammalian Cells with Environmental Chlamydiae.Infect Immun. 2020 May 20;88(6):e00851-19. doi: 10.1128/IAI.00851-19. Print 2020 May 20. Infect Immun. 2020. PMID: 32179584 Free PMC article.
-
Genome Dynamics and Temperature Adaptation During Experimental Evolution of Obligate Intracellular Bacteria.Genome Biol Evol. 2023 Aug 1;15(8):evad139. doi: 10.1093/gbe/evad139. Genome Biol Evol. 2023. PMID: 37515591 Free PMC article.
References
-
- Saraste A, Pulkki K. Morphologic and biochemical hallmarks of apoptosis. Cardiovasc Res. 2000;45:528–537. - PubMed
-
- Erwig LP, Henson PM. Clearance of apoptotic cells by phagocytes. Cell Death Differ. 2008;15:243–250. - PubMed
-
- Krysko DV, Vanden Berghe T, D'Herde K, Vandenabeele P. Apoptosis and necrosis: detection, discrimination and phagocytosis. Methods. 2008;44:205–221. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

