Regulation of the Na,K-ATPase gamma-subunit FXYD2 by Runx1 and Ret signaling in normal and injured non-peptidergic nociceptive sensory neurons
- PMID: 22253804
- PMCID: PMC3258241
- DOI: 10.1371/journal.pone.0029852
Regulation of the Na,K-ATPase gamma-subunit FXYD2 by Runx1 and Ret signaling in normal and injured non-peptidergic nociceptive sensory neurons
Abstract
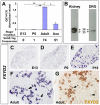
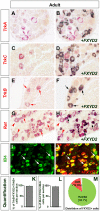
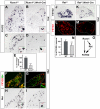
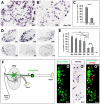
Dorsal root ganglia (DRGs) contain the cell bodies of sensory neurons which relay nociceptive, thermoceptive, mechanoceptive and proprioceptive information from peripheral tissues toward the central nervous system. These neurons establish constant communication with their targets which insures correct maturation and functioning of the somato-sensory nervous system. Interfering with this two-way communication leads to cellular, electrophysiological and molecular modifications that can eventually cause neuropathic conditions. In this study we reveal that FXYD2, which encodes the gamma-subunit of the Na,K-ATPase reported so far to be mainly expressed in the kidney, is induced in the mouse DRGs at postnatal stages where it is restricted specifically to the TrkB-expressing mechanoceptive and Ret-positive/IB4-binding non-peptidergic nociceptive neurons. In non-peptidergic nociceptors, we show that the transcription factor Runx1 controls FXYD2 expression during the maturation of the somato-sensory system, partly through regulation of the tyrosine kinase receptor Ret. Moreover, Ret signaling maintains FXYD2 expression in adults as demonstrated by the axotomy-induced down-regulation of the gene that can be reverted by in vivo delivery of GDNF family ligands. Altogether, these results establish FXYD2 as a specific marker of defined sensory neuron subtypes and a new target of the Ret signaling pathway during normal maturation of the non-peptidergic nociceptive neurons and after sciatic nerve injury.
Conflict of interest statement
Figures
Similar articles
-
p75 Is Required for the Establishment of Postnatal Sensory Neuron Diversity by Potentiating Ret Signaling.Cell Rep. 2017 Oct 17;21(3):707-720. doi: 10.1016/j.celrep.2017.09.037. Cell Rep. 2017. PMID: 29045838 Free PMC article.
-
Fxyd2 regulates Aδ- and C-fiber mechanosensitivity and is required for the maintenance of neuropathic pain.Sci Rep. 2016 Nov 2;6:36407. doi: 10.1038/srep36407. Sci Rep. 2016. PMID: 27805035 Free PMC article.
-
Tlx3 and Runx1 act in combination to coordinate the development of a cohort of nociceptors, thermoceptors, and pruriceptors.J Neurosci. 2012 Jul 11;32(28):9706-15. doi: 10.1523/JNEUROSCI.1109-12.2012. J Neurosci. 2012. PMID: 22787056 Free PMC article.
-
Essential role of Ret for defining non-peptidergic nociceptor phenotypes and functions in the adult mouse.Eur J Neurosci. 2011 Apr;33(8):1385-400. doi: 10.1111/j.1460-9568.2011.07634.x. Epub 2011 Mar 14. Eur J Neurosci. 2011. PMID: 21395865
-
Renal Mg handling, FXYD2 and the central role of the Na,K-ATPase.Physiol Rep. 2018 Sep;6(17):e13843. doi: 10.14814/phy2.13843. Physiol Rep. 2018. PMID: 30175537 Free PMC article. Review.
Cited by
-
Somatosensory neuron types identified by high-coverage single-cell RNA-sequencing and functional heterogeneity.Cell Res. 2016 Jan;26(1):83-102. doi: 10.1038/cr.2015.149. Epub 2015 Dec 22. Cell Res. 2016. PMID: 26691752 Free PMC article.
-
FXYD2, a γ subunit of Na⁺, K⁺-ATPase, maintains persistent mechanical allodynia induced by inflammation.Cell Res. 2015 Mar;25(3):318-34. doi: 10.1038/cr.2015.12. Epub 2015 Jan 30. Cell Res. 2015. PMID: 25633594 Free PMC article.
-
Hyperplasia of pancreatic beta cells and improved glucose tolerance in mice deficient in the FXYD2 subunit of Na,K-ATPase.J Biol Chem. 2013 Mar 8;288(10):7077-85. doi: 10.1074/jbc.M112.401190. Epub 2013 Jan 23. J Biol Chem. 2013. PMID: 23344951 Free PMC article.
-
FXYD2 antisense oligonucleotide provides an efficient approach for long-lasting relief of chronic peripheral pain.JCI Insight. 2023 May 8;8(9):e161246. doi: 10.1172/jci.insight.161246. JCI Insight. 2023. PMID: 37154155 Free PMC article.
-
Novel modeling of cancer cell signaling pathways enables systematic drug repositioning for distinct breast cancer metastases.Cancer Res. 2013 Oct 15;73(20):6149-63. doi: 10.1158/0008-5472.CAN-12-4617. Epub 2013 Oct 4. Cancer Res. 2013. PMID: 24097821 Free PMC article.
References
-
- Mechaly I, Bourane S, Piquemal D, Al-Jumaily M, Venteo S, et al. Gene profiling during development and after a peripheral nerve traumatism reveals genes specifically induced by injury in dorsal root ganglia. Mol Cell Neurosci. 2006;32:217–229. - PubMed
-
- Garty H, Karlish SJ. Role of FXYD proteins in ion transport. Annu Rev Physiol. 2006;68:431–459. - PubMed
-
- Geering K. FXYD proteins: new regulators of Na,K-ATPase. Am J Physiol Renal Physiol. 2006;290:F241–250. - PubMed
-
- Meij IC, Koenderink JB, van Bokhoven H, Assink KF, Groenestege WT, et al. Dominant isolated renal magnesium loss is caused by misrouting of the Na(+),K(+)-ATPase gamma-subunit. Nat Genet. 2000;26:265–266. - PubMed
-
- Kuster B, Shainskaya A, Pu HX, Goldshleger R, Blostein R, et al. A new variant of the gamma subunit of renal Na,K-ATPase. Identification by mass spectrometry, antibody binding, and expression in cultured cells. J Biol Chem. 2000;275:18441–18446. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

