The role of the multiple banded antigen of Ureaplasma parvum in intra-amniotic infection: major virulence factor or decoy?
- PMID: 22253806
- PMCID: PMC3257234
- DOI: 10.1371/journal.pone.0029856
The role of the multiple banded antigen of Ureaplasma parvum in intra-amniotic infection: major virulence factor or decoy?
Abstract
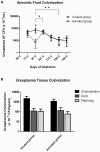
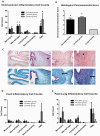
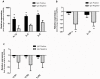
The multiple banded antigen (MBA) is a predicted virulence factor of Ureaplasma species. Antigenic variation of the MBA is a potential mechanism by which ureaplasmas avoid immune recognition and cause chronic infections of the upper genital tract of pregnant women. We tested whether the MBA is involved in the pathogenesis of intra-amniotic infection and chorioamnionitis by injecting virulent or avirulent-derived ureaplasma clones (expressing single MBA variants) into the amniotic fluid of pregnant sheep. At 55 days of gestation pregnant ewes (n = 20) received intra-amniotic injections of virulent-derived or avirulent-derived U. parvum serovar 6 strains (2×10⁴ CFU), or 10B medium (n = 5). Amniotic fluid was collected every two weeks post-infection and fetal tissues were collected at the time of surgical delivery of the fetus (140 days of gestation). Whilst chronic colonisation was established in the amniotic fluid of animals infected with avirulent-derived and virulent-derived ureaplasmas, the severity of chorioamnionitis and fetal inflammation was not different between these groups (p>0.05). MBA size variants (32-170 kDa) were generated in vivo in amniotic fluid samples from both the avirulent and virulent groups, whereas in vitro antibody selection experiments led to the emergence of MBA-negative escape variants in both strains. Anti-ureaplasma IgG antibodies were detected in the maternal serum of animals from the avirulent (40%) and virulent (55%) groups, and these antibodies correlated with increased IL-1β, IL-6 and IL-8 expression in chorioamnion tissue (p<0.05). We demonstrate that ureaplasmas are capable of MBA phase variation in vitro; however, ureaplasmas undergo MBA size variation in vivo, to potentially prevent eradication by the immune response. Size variation of the MBA did not correlate with the severity of chorioamnionitis. Nonetheless, the correlation between a maternal humoral response and the expression of chorioamnion cytokines is a novel finding. This host response may be important in the pathogenesis of inflammation-mediated adverse pregnancy outcomes.
Conflict of interest statement
Figures



Similar articles
-
Ureaplasma parvum serovar 3 multiple banded antigen size variation after chronic intra-amniotic infection/colonization.PLoS One. 2013 Apr 26;8(4):e62746. doi: 10.1371/journal.pone.0062746. Print 2013. PLoS One. 2013. PMID: 23638142 Free PMC article.
-
The severity of chorioamnionitis in pregnant sheep is associated with in vivo variation of the surface-exposed multiple-banded antigen/gene of Ureaplasma parvum.Biol Reprod. 2010 Sep;83(3):415-26. doi: 10.1095/biolreprod.109.083121. Epub 2010 Jun 2. Biol Reprod. 2010. PMID: 20519696 Free PMC article.
-
Ureaplasma Species Multiple Banded Antigen (MBA) Variation Is Associated with the Severity of Inflammation In vivo and In vitro in Human Placentae.Front Cell Infect Microbiol. 2017 Apr 13;7:123. doi: 10.3389/fcimb.2017.00123. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28451522 Free PMC article.
-
Current understanding and treatment of intra-amniotic infection with Ureaplasma spp.J Obstet Gynaecol Res. 2019 Sep;45(9):1796-1808. doi: 10.1111/jog.14052. Epub 2019 Jul 16. J Obstet Gynaecol Res. 2019. PMID: 31313469 Review.
-
The Human Ureaplasma Species as Causative Agents of Chorioamnionitis.Clin Microbiol Rev. 2016 Dec 14;30(1):349-379. doi: 10.1128/CMR.00091-16. Print 2017 Jan. Clin Microbiol Rev. 2016. PMID: 27974410 Free PMC article. Review.
Cited by
-
Ureaplasma species: role in neonatal morbidities and outcomes.Arch Dis Child Fetal Neonatal Ed. 2014 Jan;99(1):F87-92. doi: 10.1136/archdischild-2012-303351. Epub 2013 Aug 19. Arch Dis Child Fetal Neonatal Ed. 2014. PMID: 23960141 Free PMC article. Review.
-
Augmented Th17-type immune responses in preterm neonates exposed to histologic chorioamnionitis.Pediatr Res. 2017 Apr;81(4):639-645. doi: 10.1038/pr.2016.254. Epub 2016 Nov 21. Pediatr Res. 2017. PMID: 27870827 Free PMC article.
-
Immune profiling of BALB/C and C57BL/6 mice reveals a correlation between Ureaplasma parvum-Induced fetal inflammatory response syndrome-like pathology and increased placental expression of TLR2 and CD14.Am J Reprod Immunol. 2014 Mar;71(3):241-51. doi: 10.1111/aji.12192. Epub 2013 Dec 27. Am J Reprod Immunol. 2014. PMID: 24372928 Free PMC article.
-
Host genetic background impacts disease outcome during intrauterine infection with Ureaplasma parvum.PLoS One. 2012;7(8):e44047. doi: 10.1371/journal.pone.0044047. Epub 2012 Aug 29. PLoS One. 2012. PMID: 22952869 Free PMC article.
-
Infection strategies of mycoplasmas: Unraveling the panoply of virulence factors.Virulence. 2021 Dec;12(1):788-817. doi: 10.1080/21505594.2021.1889813. Virulence. 2021. PMID: 33704021 Free PMC article. Review.
References
-
- Robertson JA, Stemke GW, Davis JW, Jr, Harasawa R, Thirkell D, et al. Proposal of Ureaplasma parvum sp. nov. and emended description of Ureaplasma urealyticum (Shepard et al. 1974) Robertson et al. 2001. Int J Syst Evol Microbiol. 2002;52:587–597. - PubMed
-
- Glass JI, Lefkowitz EJ, Glass JS, Heiner CR, Chen EY, et al. The complete sequence of the mucosal pathogen Ureaplasma urealyticum. Nature. 2000;407:757–762. - PubMed
-
- Volgmann T, Ohlinger R, Panzig B. Ureaplasma urealyticum-harmless commensal or underestimated enemy of human reproduction? A review. Arch Gynecol Obstet. 2005;273:133–139. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

