Persistence of Borrelia burgdorferi in rhesus macaques following antibiotic treatment of disseminated infection
- PMID: 22253822
- PMCID: PMC3256191
- DOI: 10.1371/journal.pone.0029914
Persistence of Borrelia burgdorferi in rhesus macaques following antibiotic treatment of disseminated infection
Erratum in
- PLoS One. 2012;7(4):10.1371/annotation/4cafed66-fb84-4589-a001-131d9c50aea6
- PLoS One. 2013;8(9). doi:10.1371/annotation/f84663e3-0a2c-4243-8f97-3a58133c1b0f
-
Correction: Persistence of Borrelia burgdorferi in Rhesus Macaques following Antibiotic Treatment of Disseminated Infection.PLoS One. 2013 Sep 24;8(9):10.1371/annotation/f84663e3-0a2c-4243-8f97-3a58133c1b0f. doi: 10.1371/annotation/f84663e3-0a2c-4243-8f97-3a58133c1b0f. eCollection 2013. PLoS One. 2013. PMID: 24116243 Free PMC article.
Abstract
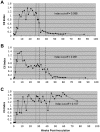
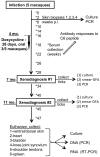
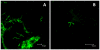
The persistence of symptoms in Lyme disease patients following antibiotic therapy, and their causes, continue to be a matter of intense controversy. The studies presented here explore antibiotic efficacy using nonhuman primates. Rhesus macaques were infected with B. burgdorferi and a portion received aggressive antibiotic therapy 4-6 months later. Multiple methods were utilized for detection of residual organisms, including the feeding of lab-reared ticks on monkeys (xenodiagnosis), culture, immunofluorescence and PCR. Antibody responses to the B. burgdorferi-specific C6 diagnostic peptide were measured longitudinally and declined in all treated animals. B. burgdorferi antigen, DNA and RNA were detected in the tissues of treated animals. Finally, small numbers of intact spirochetes were recovered by xenodiagnosis from treated monkeys. These results demonstrate that B. burgdorferi can withstand antibiotic treatment, administered post-dissemination, in a primate host. Though B. burgdorferi is not known to possess resistance mechanisms and is susceptible to the standard antibiotics (doxycycline, ceftriaxone) in vitro, it appears to become tolerant post-dissemination in the primate host. This finding raises important questions about the pathogenicity of antibiotic-tolerant persisters and whether or not they can contribute to symptoms post-treatment.
Conflict of interest statement
Figures



References
-
- Wormser Gary P, Dattwyler Raymond J, Shapiro Eugene D, Halperin John J, Steere A, et al. The Clinical Assessment, Treatment, and Prevention of Lyme Disease, Human Granulocytic Anaplasmosis, and Babesiosis: Clinical Practice Guidelines by the Infectious Diseases Society of America. Clinical Infectious Diseases. 2006;43:1089–1134. - PubMed
-
- Asch ES, Bujak DI, Weiss M, Peterson MG, Weinstein A. Lyme disease: an infectious and postinfectious syndrome. Journal of Rheumatology. 1994;21:454–461. - PubMed
-
- Shadick NA, Phillips CB, Logigian EL, Steere AC, Kaplan RF, et al. The long-term clinical outcomes of Lyme disease. A population-based retrospective cohort study. Annals of Internal Medicine. 1994;121:560–567. - PubMed
-
- Steere AC, Levin RE, Molloy PJ, Kalish RA, Abraham JH, 3rd, et al. Treatment of Lyme arthritis. Arthritis & Rheumatism. 1994;37:878–888. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

