Epigallocatechin-3-gallate as a potential therapeutic drug for TTR-related amyloidosis: "in vivo" evidence from FAP mice models
- PMID: 22253829
- PMCID: PMC3254632
- DOI: 10.1371/journal.pone.0029933
Epigallocatechin-3-gallate as a potential therapeutic drug for TTR-related amyloidosis: "in vivo" evidence from FAP mice models
Abstract
Background: Familial amyloidotic polyneuropathy (FAP) is a neurodegenerative disease caused by the extracellular deposition of mutant transthyretin (TTR), with special involvement of the peripheral nervous system (PNS). Currently, hepatic transplantation is considered the most efficient therapy to halt the progression of clinical symptoms in FAP since more than 95% of TTR is produced by the liver. However, less invasive and more reliable therapeutic approaches have been proposed for FAP therapy, namely based on drugs acting as inhibitors of amyloid formation or as amyloid disruptors. We have recently reported that epigallocatechin-3-gallate (EGCG), the most abundant catechin in green tea, is able to inhibit TTR aggregation and fibril formation, "in vitro" and in a cellular system, and is also able to disrupt pre-formed amyloid fibrils "in vitro".
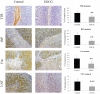
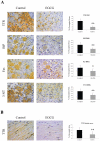
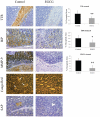
Methodology and principal findings: In the present study, we assessed the effect of EGCG subchronic administration on TTR amyloidogenesis "in vivo", using well characterized animal models for FAP. Semiquantitative immunohistochemistry (SQ-IHC) and Western blot analysis of mice tissues after treatment demonstrated that EGCG inhibits TTR toxic aggregates deposition in about 50% along the gastrointestinal tract (GI) and peripheral nervous system (PNS). Moreover EGCG treatment considerably lowered levels of several biomarkers associated with non-fibrillar TTR deposition, namely endoplasmic reticulum (ER)-stress, protein oxidation and apoptosis markers. Treatment of old FAP mice with EGCG resulted not only in the decrease of non-fibrillar TTR deposition but also in disaggregation of amyloid deposits. Consistently, matrix metalloproteinase (MMP)-9 and serum amyloid P component (SAP), both markers of amyloid deposition, were also found reduced in treated old FAP mice.
Conclusions and significance: The dual effect of EGCG both as TTR aggregation inhibitor and amyloid fibril disruptor together with the high tolerability and low toxicity of EGCG in humans, point towards the potential use of this compound, or optimized derivatives, in the treatment of TTR-related amyloidoses.
Conflict of interest statement
Figures



References
-
- Benson MD, Kincaid JC. The molecular biology and clinical features of amyloid neuropathy. Muscle Nerve. 2007;36(4):411–23. - PubMed
-
- Quintas A, Vaz DC, Cardoso I, Saraiva MJ, Brito RM. Tetramer dissociation and monomer partial unfolding precedes protofibril formation in amyloidogenic transthyretin variants. J Biol Chem. 2001;276(29):27207–13. - PubMed
-
- Cardoso I, Goldsbury CS, Müller SA, Olivieri V, Wirtz S, et al. Transthyretin fibrillogenesis entails the assembly of monomers: a molecular model for in vitro assembled transthyretin amyloid-like fibrils. J Mol Biol. 2002;317(5):683–95. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

