A2BR adenosine receptor modulates sweet taste in circumvallate taste buds
- PMID: 22253866
- PMCID: PMC3254652
- DOI: 10.1371/journal.pone.0030032
A2BR adenosine receptor modulates sweet taste in circumvallate taste buds
Abstract
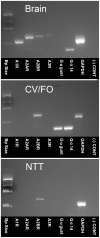
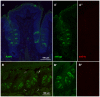
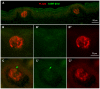
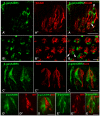
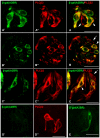
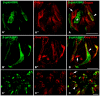
In response to taste stimulation, taste buds release ATP, which activates ionotropic ATP receptors (P2X2/P2X3) on taste nerves as well as metabotropic (P2Y) purinergic receptors on taste bud cells. The action of the extracellular ATP is terminated by ectonucleotidases, ultimately generating adenosine, which itself can activate one or more G-protein coupled adenosine receptors: A1, A2A, A2B, and A3. Here we investigated the expression of adenosine receptors in mouse taste buds at both the nucleotide and protein expression levels. Of the adenosine receptors, only A2B receptor (A2BR) is expressed specifically in taste epithelia. Further, A2BR is expressed abundantly only in a subset of taste bud cells of posterior (circumvallate, foliate), but not anterior (fungiform, palate) taste fields in mice. Analysis of double-labeled tissue indicates that A2BR occurs on Type II taste bud cells that also express Gα14, which is present only in sweet-sensitive taste cells of the foliate and circumvallate papillae. Glossopharyngeal nerve recordings from A2BR knockout mice show significantly reduced responses to both sucrose and synthetic sweeteners, but normal responses to tastants representing other qualities. Thus, our study identified a novel regulator of sweet taste, the A2BR, which functions to potentiate sweet responses in posterior lingual taste fields.
Conflict of interest statement
Figures


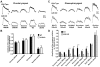
References
-
- Finger TE, Danilova V, Barrows J, Bartel DL, Vigers AJ, et al. ATP signaling is crucial for communication from taste buds to gustatory nerves. Science. 2005;310:1495–1499. - PubMed
-
- Bo X, Alavi A, Xiang Z, Oglesby I, Ford A, et al. Localization of ATP-gated P2X2 and P2X3 receptor immunoreactive nerves in rat taste buds. Neuroreport. 1999;10:1107–1111. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

