Rescue of dystrophic skeletal muscle by PGC-1α involves a fast to slow fiber type shift in the mdx mouse
- PMID: 22253880
- PMCID: PMC3256197
- DOI: 10.1371/journal.pone.0030063
Rescue of dystrophic skeletal muscle by PGC-1α involves a fast to slow fiber type shift in the mdx mouse
Abstract
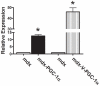
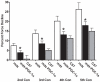
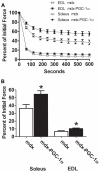
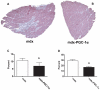
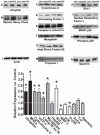
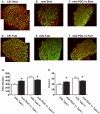
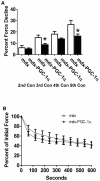
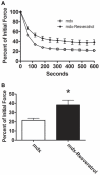
Increased utrophin expression is known to reduce pathology in dystrophin-deficient skeletal muscles. Transgenic over-expression of PGC-1α has been shown to increase levels of utrophin mRNA and improve the histology of mdx muscles. Other reports have shown that PGC-1α signaling can lead to increased oxidative capacity and a fast to slow fiber type shift. Given that it has been shown that slow fibers produce and maintain more utrophin than fast skeletal muscle fibers, we hypothesized that over-expression of PGC-1α in post-natal mdx mice would increase utrophin levels via a fiber type shift, resulting in more slow, oxidative fibers that are also more resistant to contraction-induced damage. To test this hypothesis, neonatal mdx mice were injected with recombinant adeno-associated virus (AAV) driving expression of PGC-1α. PGC-1α over-expression resulted in increased utrophin and type I myosin heavy chain expression as well as elevated mitochondrial protein expression. Muscles were shown to be more resistant to contraction-induced damage and more fatigue resistant. Sirt-1 was increased while p38 activation and NRF-1 were reduced in PGC-1α over-expressing muscle when compared to control. We also evaluated if the use a pharmacological PGC-1α pathway activator, resveratrol, could drive the same physiological changes. Resveratrol administration (100 mg/kg/day) resulted in improved fatigue resistance, but did not achieve significant increases in utrophin expression. These data suggest that the PGC-1α pathway is a potential target for therapeutic intervention in dystrophic skeletal muscle.
Conflict of interest statement
Figures
References
-
- Hopf FW, Turner PR, Steinhardt RA. Calcium misregulation and the pathogenesis of muscular dystrophy. Subcell Biochem. 2007;45:429–464. - PubMed
-
- Whitehead NP, Yeung EW, Allen DG. Muscle damage in mdx (dystrophic) mice: role of calcium and reactive oxygen species. Clin Exp Pharmacol Physiol. 2006;33:657–662. - PubMed
-
- Spencer MJ, Croall DE, Tidball JG. Calpains are activated in necrotic fibers from mdx dystrophic mice. J Biol Chem. 1995;270:10909–10914. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

