HIV-1 promotes renal tubular epithelial cell protein synthesis: role of mTOR pathway
- PMID: 22253885
- PMCID: PMC3253808
- DOI: 10.1371/journal.pone.0030071
HIV-1 promotes renal tubular epithelial cell protein synthesis: role of mTOR pathway
Abstract
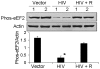
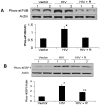
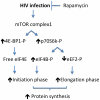
Tubular cell HIV-infection has been reported to manifest in the form of cellular hypertrophy and apoptosis. In the present study, we evaluated the role of mammalian target of rapamycin (mTOR) pathway in the HIV induction of tubular cell protein synthesis. Mouse proximal tubular epithelial cells (MPTECs) were transduced with either gag/pol-deleted NL4-3 (HIV/MPTEC) or empty vector (Vector/MPTEC). HIV/MPTEC showed enhanced DNA synthesis when compared with Vector/MPTECs by BRDU labeling studies. HIV/MPTECs also showed enhanced production of β-laminin and fibronection in addition to increased protein content per cell. In in vivo studies, renal cortical sections from HIV transgenic mice and HIVAN patients showed enhanced tubular cell phosphorylation of mTOR. Analysis of mTOR revealed increased expression of phospho (p)-mTOR in HIV/MPTECs when compared to vector/MPTECs. Further downstream analysis of mTOR pathway revealed enhanced phosphorylation of p70S6 kinase and associated diminished phosphorylation of eEF2 (eukaryotic translation elongation factor 2) in HIV/MPTECs; moreover, HIV/MPTECs displayed enhanced phosphorylation of eIF4B (eukaryotic translation initiation factor 4B) and 4EBP-1 (eukaryotic 4E binding protein). To confirm our hypothesis, we evaluated the effect of rapamycin on HIV-induced tubular cell downstream signaling. Rapamycin not only attenuated phosphorylation of p70S6 kinase and associated down stream signaling in HIV/MPTECs but also inhibited HIV-1 induced tubular cell protein synthesis. These findings suggest that mTOR pathway is activated in HIV-induced enhanced tubular cell protein synthesis and contributes to tubular cell hypertrophy.
Conflict of interest statement
Figures







References
-
- Szczech LA, Gupta SK, Habash R, Guasch A, Kalayjian R, et al. The clinical epidemiology and course of the spectrum of renal diseases associated with HIV infection. Kidney Int. 2004;66:1145–52. - PubMed
-
- Tao Y, Kim J, Schrier RW, Edelstein CL. Rapamycin markedly slows disease progression in a rat model of polycystic kidney disease. J Am Soc Nephrol. 2005;16:46–51. - PubMed
-
- Wu M, Arcaro A, Varga Z, Vogetseder A, Le Hir M, et al. Pulse mTOR inhibitor treatment effectively controls cyst growth but leads to severe parenchymal and glomerular hypertrophy in rat polycystic kidney disease. Am J Physiol Renal Physiol. 2009;297(6):F1597–605. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

