Region specific and worldwide distribution of collagen-binding M proteins with PARF motifs among human pathogenic streptococcal isolates
- PMID: 22253902
- PMCID: PMC3256231
- DOI: 10.1371/journal.pone.0030122
Region specific and worldwide distribution of collagen-binding M proteins with PARF motifs among human pathogenic streptococcal isolates
Abstract
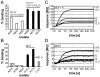
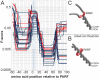
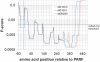
Some of the variety of Streptococcus pyogenes and Streptococcus dysgalactiae ssp. equisimilis (SDSE) M proteins act as collagen-binding adhesins that facilitate acute infection. Moreover, their potential to trigger collagen autoimmunity has been implicated in the pathogenesis of acute rheumatic fever and attributed to a collagen-binding motif called PARF (peptide associated with rheumatic fever). For the first time we determine the rate of clinical isolates with collagen-binding M proteins that use a PARF motif (A/T/E)XYLXX(L/F)N in a defined geographic region, Vellore in South India. In this region both, incidence of streptococcal infections and prevalence of acute rheumatic fever are high. M proteins with PARF motif conferred collagen-binding activity to 3.9% of 153 S. pyogenes and 10.6% of 255 SDSE clinical isolates from Vellore. The PARF motif occurred in three S. pyogenes and 22 SDSE M protein types. In one of the S. pyogenes and five of the SDSE M proteins that contained the motif, collagen-binding was impaired, due to influences of other parts of the M protein molecule. The accumulated data on the collagen binding activity of certain M protein types allowed a reanalysis of published worldwide emm-typing data with the aim to estimate the rates of isolates that bind collagen via PARF. The results indicate that M proteins, which bind collagen via a PARF motif, are epidemiologically relevant in human infections, not only in Vellore. It is imperative to include the most relevant collagen-binding M types in vaccines. But when designing M protein based vaccines it should be considered that collagen binding motifs within the vaccine antigen remain potential risk factors.
Conflict of interest statement
Figures

References
-
- Efstratiou A. Pyogenic streptococci of Lancefield groups C and G as pathogens in man. Soc Appl Bacteriol Symp Ser. 1997;26:72S–79S. - PubMed
-
- Baracco GJ, Bisno AL. Group C and group G streptococcal infections: epidemiologic and clinical aspects. In: Fischetti VA, editor. Gram-positive pathogens. 2nd ed. Washington, D.C.: ASM Press; 2006. pp. 223–229.
-
- Haidan A, Talay SR, Rohde M, Sriprakash KS, Currie BJ, et al. Pharyngeal carriage of group C and group G streptococci and acute rheumatic fever in an Aboriginal population. Lancet. 2000;356:1167–1169. - PubMed
-
- Wannamaker LW. The chain that links the heart to the throat. Circulation. 1973;48:9–18. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

