Immunological evaluation of lipopeptide group A streptococcus (GAS) vaccine: structure-activity relationship
- PMID: 22253911
- PMCID: PMC3257266
- DOI: 10.1371/journal.pone.0030146
Immunological evaluation of lipopeptide group A streptococcus (GAS) vaccine: structure-activity relationship
Abstract
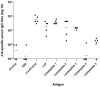
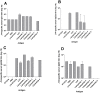
Streptococcus pyogenes (group A streptococcus, GAS) is a Gram-positive bacterial pathogen responsible for a wide variety of diseases. To date, GAS vaccine development has focused primarily on the M-protein. The M-protein is highly variable at the amino (N)-terminus (determining serotype) but is conserved at the carboxyl (C)-terminus. Previously a 29 amino acid peptide (named J14) from the conserved region of the M-protein was identified as a potential vaccine candidate. J14 was capable of eliciting protective antibodies that recognized many GAS serotypes when co-administered with immuno-stimulants. This minimal epitope however showed no immunogenicity when administered alone. In an attempt overcome this immunological non-responsiveness, we developed a self-adjuvanting vaccine candidate composed of three components: the B-cell epitope (J14), a universal helper T-cell epitope (P25) and a lipid moiety consisting of lipoamino acids (Laas) which target Toll-like receptor 2 (TLR2). Immunological evaluation in B10.BR (H-2k) mice demonstrated that the epitope attachment to the point of lipid moiety, and the length of the Laa alkyl chain have a profound effect on vaccine immunogenicity after intranasal administration. It was demonstrated that a vaccine featuring C-terminal lipid moiety containing alkyl chains of 16 carbons, with P25 located at the N-terminus, and J14 attached to the side chain of a central lysine residue was capable of inducing optimal antibody response. These findings have considerable relevance to the development of a broad spectrum J14-based GAS vaccine and in particular provided a rational basis for peptide vaccine design based on this self-adjuvanting lipopeptide technology.
Conflict of interest statement
Figures



Similar articles
-
Structure-activity relationship of lipopeptide Group A streptococcus (GAS) vaccine candidates on toll-like receptor 2.Vaccine. 2010 Mar 2;28(10):2243-2248. doi: 10.1016/j.vaccine.2009.12.046. Epub 2009 Dec 31. Vaccine. 2010. PMID: 20045502
-
Design of three-component vaccines against group A streptococcal infections: importance of spatial arrangement of vaccine components.J Med Chem. 2010 Nov 25;53(22):8041-6. doi: 10.1021/jm1007787. Epub 2010 Oct 28. J Med Chem. 2010. PMID: 21028828
-
Structure-activity relationship for the development of a self-adjuvanting mucosally active lipopeptide vaccine against Streptococcus pyogenes.J Med Chem. 2012 Oct 11;55(19):8515-23. doi: 10.1021/jm301074n. Epub 2012 Sep 25. J Med Chem. 2012. PMID: 22974133
-
Towards the development of a broadly protective group a streptococcal vaccine based on the Lipid-Core Peptide system.Curr Med Chem. 2007;14(28):2976-88. doi: 10.2174/092986707782794069. Curr Med Chem. 2007. PMID: 18220734 Review.
-
Moving forward: a mucosal vaccine against group A streptococcus.Expert Rev Vaccines. 2009 Jun;8(6):747-60. doi: 10.1586/erv.09.33. Expert Rev Vaccines. 2009. PMID: 19485755 Review.
Cited by
-
Strategies for intranasal delivery of vaccines.Drug Deliv Transl Res. 2013 Feb;3(1):100-9. doi: 10.1007/s13346-012-0085-z. Epub 2012 Jul 12. Drug Deliv Transl Res. 2013. PMID: 23316448 Free PMC article.
-
Identifying protective Streptococcus pyogenes vaccine antigens recognized by both B and T cells in human adults and children.Sci Rep. 2016 Feb 25;6:22030. doi: 10.1038/srep22030. Sci Rep. 2016. PMID: 26911649 Free PMC article.
-
Correlates of Protection for M Protein-Based Vaccines against Group A Streptococcus.J Immunol Res. 2015;2015:167089. doi: 10.1155/2015/167089. Epub 2015 May 25. J Immunol Res. 2015. PMID: 26101780 Free PMC article. Review.
-
Application of built-in adjuvants for epitope-based vaccines.PeerJ. 2019 Jan 14;6:e6185. doi: 10.7717/peerj.6185. eCollection 2019. PeerJ. 2019. PMID: 30656066 Free PMC article.
-
Lipopeptides for Vaccine Development.Bioconjug Chem. 2021 Aug 18;32(8):1472-1490. doi: 10.1021/acs.bioconjchem.1c00258. Epub 2021 Jul 6. Bioconjug Chem. 2021. PMID: 34228433 Free PMC article. Review.
References
-
- Metzgar D, McDonough EA, Hansen CJ, Blaesing CR, Baynes D, et al. Local changes in rates of group A Streptococcus disease and antibiotic resistance are associated with geographically widespread strain turnover events. Virulence. 2010;1:247–253. - PubMed
-
- Steer AC, Carapetis JR. Acute rheumatic fever and rheumatic heart disease in indigenous populations. Pediatr Clin North Am. 2009;56:1401–1419. - PubMed
-
- Olive C, Batzloff MR, Toth I. Lipid core peptide technology and group A streptococcal vaccine delivery. Expert Rev Vaccines. 2004;3:43–58. - PubMed
-
- Robinson JH, Kehoe MA. Group A streptococcal M proteins: virulence factors and protective antigens. Immunol Today. 1992;13:362–367. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

