Diagnostic accuracy of molecular amplification tests for human African trypanosomiasis--systematic review
- PMID: 22253934
- PMCID: PMC3254661
- DOI: 10.1371/journal.pntd.0001438
Diagnostic accuracy of molecular amplification tests for human African trypanosomiasis--systematic review
Abstract
Background: A range of molecular amplification techniques have been developed for the diagnosis of Human African Trypanosomiasis (HAT); however, careful evaluation of these tests must precede implementation to ensure their high clinical accuracy. Here, we investigated the diagnostic accuracy of molecular amplification tests for HAT, the quality of articles and reasons for variation in accuracy.
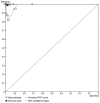
Methodology: Data from studies assessing diagnostic molecular amplification tests were extracted and pooled to calculate accuracy. Articles were included if they reported sensitivity and specificity or data whereby values could be calculated. Study quality was assessed using QUADAS and selected studies were analysed using the bivariate random effects model.
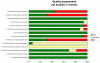
Results: 16 articles evaluating molecular amplification tests fulfilled the inclusion criteria: PCR (n = 12), NASBA (n = 2), LAMP (n = 1) and a study comparing PCR and NASBA (n = 1). Fourteen articles, including 19 different studies were included in the meta-analysis. Summary sensitivity for PCR on blood was 99.0% (95% CI 92.8 to 99.9) and the specificity was 97.7% (95% CI 93.0 to 99.3). Differences in study design and readout method did not significantly change estimates although use of satellite DNA as a target significantly lowers specificity. Sensitivity and specificity of PCR on CSF for staging varied from 87.6% to 100%, and 55.6% to 82.9% respectively.
Conclusion: Here, PCR seems to have sufficient accuracy to replace microscopy where facilities allow, although this conclusion is based on multiple reference standards and a patient population that was not always representative. Future studies should, therefore, include patients for which PCR may become the test of choice and consider well designed diagnostic accuracy studies to provide extra evidence on the value of PCR in practice. Another use of PCR for control of disease could be to screen samples collected from rural areas and test in reference laboratories, to spot epidemics quickly and direct resources appropriately.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
Rapid, point-of-care antigen tests for diagnosis of SARS-CoV-2 infection.Cochrane Database Syst Rev. 2022 Jul 22;7(7):CD013705. doi: 10.1002/14651858.CD013705.pub3. Cochrane Database Syst Rev. 2022. PMID: 35866452 Free PMC article.
-
Diagnostic test accuracy and cost-effectiveness of tests for codeletion of chromosomal arms 1p and 19q in people with glioma.Cochrane Database Syst Rev. 2022 Mar 2;3(3):CD013387. doi: 10.1002/14651858.CD013387.pub2. Cochrane Database Syst Rev. 2022. PMID: 35233774 Free PMC article.
-
Blood biomarkers for the non-invasive diagnosis of endometriosis.Cochrane Database Syst Rev. 2016 May 1;2016(5):CD012179. doi: 10.1002/14651858.CD012179. Cochrane Database Syst Rev. 2016. PMID: 27132058 Free PMC article.
-
Antibody tests for identification of current and past infection with SARS-CoV-2.Cochrane Database Syst Rev. 2022 Nov 17;11(11):CD013652. doi: 10.1002/14651858.CD013652.pub2. Cochrane Database Syst Rev. 2022. PMID: 36394900 Free PMC article.
Cited by
-
Important hemoprotozoan diseases of livestock: Challenges in current diagnostics and therapeutics: An update.Vet World. 2016 May;9(5):487-95. doi: 10.14202/vetworld.2016.487-495. Epub 2016 May 20. Vet World. 2016. PMID: 27284225 Free PMC article. Review.
-
A 5-Year Prospective Study on Incidence and Clinico-pathological Changes Associated with Naturally Occurring Trypanosomosis in Dogs of Mizoram, India.Acta Parasitol. 2022 Mar;67(1):61-71. doi: 10.1007/s11686-021-00425-0. Epub 2021 Jun 17. Acta Parasitol. 2022. PMID: 34138413
-
Comparison of loop-mediated isothermal amplification (LAMP) and PCR for the diagnosis of infection with Trypanosoma brucei ssp. in equids in The Gambia.PLoS One. 2020 Aug 24;15(8):e0237187. doi: 10.1371/journal.pone.0237187. eCollection 2020. PLoS One. 2020. PMID: 32833981 Free PMC article.
-
Prevalence of Trypanosoma congolense and Trypanosoma vivax in Lira District, Uganda.Biomed Res Int. 2021 Jun 14;2021:7284042. doi: 10.1155/2021/7284042. eCollection 2021. Biomed Res Int. 2021. PMID: 34222483 Free PMC article.
-
Diagnostic accuracy of loopamp Trypanosoma brucei detection kit for diagnosis of human African trypanosomiasis in clinical samples.PLoS Negl Trop Dis. 2013 Oct 17;7(10):e2504. doi: 10.1371/journal.pntd.0002504. eCollection 2013. PLoS Negl Trop Dis. 2013. PMID: 24147176 Free PMC article.
References
-
- Deborggraeve S, Buscher P. Molecular diagnostics for sleeping sickness: what is the benefit for the patient? Lancet Infect Dis. 2010;10:433–439. - PubMed
-
- Woo PT. The haematocrit centrifuge technique for the diagnosis of African trypanosomiasis. Acta Trop. 1970;27:384–386. - PubMed
-
- Bailey JW, Smith DH. The quantitative buffy coat for the diagnosis of trypanosomes. Trop Doct. 1994;24:54–56. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

