Extending vaterite microviscometry to ex vivo blood vessels by serial calibration
- PMID: 22254166
- PMCID: PMC3255340
- DOI: 10.1364/BOE.3.000037
Extending vaterite microviscometry to ex vivo blood vessels by serial calibration
Abstract
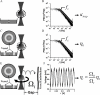
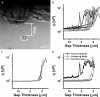
The endothelial glycocalyx layer is a ~2 µm thick glycosaminoglycan rich pericellular matrix expressed on the luminal surface of vascular endothelial cells, which has implications in vessel mechanics and mechanotransduction. Despite its role in vascular physiology, no direct measurement has of yet been made of vessel glycocalyx material properties. Vaterite microviscometry is a laser tweezers based microrheological method, which has been previously utilized to measure the viscosity of linear and complex fluids under flow. This form of microrheology has until now relied on complete recollection of the forward scattered light. Here we present a novel method to extend vaterite microviscometry to relatively thick samples. We validate our method and its assumptions and measure the apparent viscosity as a function of distance from the vascular endothelium. We observe a differential response in conditions designed to preserve the EGL in comparison to those designed to collapse it.
Keywords: (140.7010) Laser trapping; (160.1435) Biomaterials; (170.4520) Optical confinement and manipulation.
2011 Optical Society of America
Figures

References
-
- Vink H., Duling B. R., “Identification of distinct luminal domains for macromolecules, erythrocytes, and leukocytes within mammalian capillaries,” Circ. Res. 79(3), 581–589 (1996). - PubMed
