Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial
- PMID: 22257673
- PMCID: PMC5705192
- DOI: 10.1016/S0140-6736(11)61847-3
Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial
Erratum in
- Lancet. 2012 Feb 18;379(9816):616. Dosage error in published abstract; MEDLINE/PubMed abstract corrected
Abstract
Background: The anti-HER2 monoclonal antibody trastuzumab and the tyrosine kinase inhibitor lapatinib have complementary mechanisms of action and synergistic antitumour activity in models of HER2-overexpressing breast cancer. We argue that the two anti-HER2 agents given together would be better than single-agent therapy.
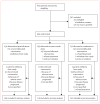
Methods: In this parallel groups, randomised, open-label, phase 3 study undertaken between Jan 5, 2008, and May 27, 2010, women from 23 countries with HER2-positive primary breast cancer with tumours greater than 2 cm in diameter were randomly assigned to oral lapatinib (1500 mg), intravenous trastuzumab (loading dose 4 mg/kg [DOSAGE ERROR CORRECTED], subsequent doses 2 mg/kg), or lapatinib (1000 mg) plus trastuzumab. Treatment allocation was by stratified, permuted blocks randomisation, with four stratification factors. Anti-HER2 therapy alone was given for the first 6 weeks; weekly paclitaxel (80 mg/m(2)) was then added to the regimen for a further 12 weeks, before definitive surgery was undertaken. After surgery, patients received adjuvant chemotherapy followed by the same targeted therapy as in the neoadjuvant phase to 52 weeks. The primary endpoint was the rate of pathological complete response (pCR), analysed by intention to treat. This trial is registered with ClinicalTrials.gov, NCT00553358.
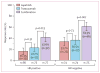
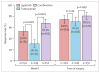
Findings: 154 patients received lapatinib, 149 trastuzumab, and 152 the combination. pCR rate was significantly higher in the group given lapatinib and trastuzumab (78 of 152 patients [51·3%; 95% CI 43·1-59·5]) than in the group given trastuzumab alone (44 of 149 patients [29·5%; 22·4-37·5]; difference 21·1%, 9·1-34·2, p=0·0001). We recorded no significant difference in pCR between the lapatinib (38 of 154 patients [24·7%, 18·1-32·3]) and the trastuzumab (difference -4·8%, -17·6 to 8·2, p=0·34) groups. No major cardiac dysfunctions occurred. Frequency of grade 3 diarrhoea was higher with lapatinib (36 patients [23·4%]) and lapatinib plus trastuzumab (32 [21·1%]) than with trastuzumab (three [2·0%]). Similarly, grade 3 liver-enzyme alterations were more frequent with lapatinib (27 [17·5%]) and lapatinib plus trastuzumab (15 [9·9%]) than with trastuzumab (11 [7·4%]).
Interpretation: Dual inhibition of HER2 might be a valid approach to treatment of HER2-positive breast cancer in the neoadjuvant setting.
Funding: GlaxoSmithKline.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Conflict of interest statement
JB has received honoraria from Roche. IB’s institution has received funding from GlaxoSmithKline and Roche. HE has been a speaker and received travel grants from GlaxoSmithKline. SDC has been a speaker for GlaxoSmithKline. EdA has served on an advisory board and received a travelling grant from GlaxoSmithKline, and has been a speaker for Roche. KF’s institution has received travelling grants from GlaxoSmithKline. VVD’s and T-WC’s institutions have received research funding from GlaxoSmithKline. AG has received honoraria from GlaxoSmithKline and Roche. T-WC has been a speaker for GlaxoSmithKline, Roche, Novartis, and Amgen; and has received consultancy funding from GlaxoSmithKline, Roche, Abbott, AstraZeneca, Novartis, and Amgen. LP has received consultancy fees from Pfizer, Sanofi, and Bristol-Myers Squibb; and research grants from Bristol-Myers Squibb and AstraZeneca. RDG’s institution has received research funding from GlaxoSmithKline and Roche. MP-G has received honoraria from GlaxoSmithKline and Roche, and her institution has received research funding from GlaxoSmithKline. GA is a salaried employee of GlaxoSmithKline and retains stock and stock options at the company. The other authors declare that they have no conflicts of interest.
Figures

Comment in
-
Dual inhibition of HER2 in breast cancer treatment.Lancet. 2012 Feb 18;379(9816):596-8. doi: 10.1016/S0140-6736(12)60068-3. Epub 2012 Jan 17. Lancet. 2012. PMID: 22257672 No abstract available.
-
Neoadjuvant chemotherapy with HER2 inhibitors for breast cancer.Lancet. 2012 Jun 16;379(9833):2237; author reply 2238. doi: 10.1016/S0140-6736(12)60970-2. Lancet. 2012. PMID: 22704160 No abstract available.
-
Neoadjuvant chemotherapy with HER2 inhibitors for breast cancer.Lancet. 2012 Jun 16;379(9833):2237; author reply 2238. doi: 10.1016/S0140-6736(12)60971-4. Lancet. 2012. PMID: 22704161 No abstract available.
References
-
- Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer. 2005;5:341–54. - PubMed
-
- Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–92. - PubMed
-
- Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–84. - PubMed
-
- Smith I, Procter M, Gelber RD, et al. for the HERA study team. 2-year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: a randomised controlled trial. Lancet. 2007;369:29–36. - PubMed
-
- Joensuu H, Kellokumpu-Lehtinen PL, Bono P, et al. the FinHer Study Investigators. Adjuvant docetaxel or vinorelbine with or without trastuzumab for breast cancer. N Engl J Med. 2006;354:809–20. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

