Changes in the frequency and in vivo vessel-forming ability of rhesus monkey circulating endothelial colony-forming cells across the lifespan (birth to aged)
- PMID: 22258126
- PMCID: PMC3358134
- DOI: 10.1038/pr.2011.22
Changes in the frequency and in vivo vessel-forming ability of rhesus monkey circulating endothelial colony-forming cells across the lifespan (birth to aged)
Abstract
Introduction: We have identified a novel hierarchy of human endothelial colony-forming cells (ECFCs) that are functionally defined by their proliferative and clonogenic potential and in vivo vessel-forming ability. The rhesus monkey provides an excellent model in which to examine the changes in circulating concentrations and functions of ECFCs since this nonhuman primate possesses a long lifespan and has been used extensively to model age-related processes that occur in humans.
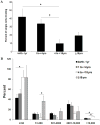
Results: Endothelial cells (ECs) derived from rhesus monkey ECFCs share a cell-surface phenotype similar to human cord blood ECFCs, rapidly form capillary-like structures in vitro, and form endothelial-lined vessels in vivo upon implantation in immunodeficient mice in an age-dependent manner. Of interest, although ECFCs from the oldest monkeys formed capillary-like structures in vitro, the cells failed to form inosculating vessels when implanted in vivo and displayed a deficiency in cytoplasmic vacuolation in vitro; a critical first step in vasculogenesis.
Discussion: Utilizing previously established clonogenic assays for defining different subpopulations of human ECFCs, we have shown that a hierarchy of ECFCs, identical to human cells, can be isolated from the peripheral blood of rhesus monkeys, and that the frequency of the circulating cells varies with age. These studies establish the rhesus monkey as an important preclinical model for evaluating the role and function of circulating ECFCs in vascular homeostasis and aging.
Methods: Peripheral blood samples were collected from 40 healthy rhesus monkeys from birth to 24 years of age for ECFC analysis including immunophenotyping, clonogenic assays, and in vivo vessel formation.
Figures



References
-
- Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

