HIV cell-to-cell transmission requires the production of infectious virus particles and does not proceed through env-mediated fusion pores
- PMID: 22258237
- PMCID: PMC3302491
- DOI: 10.1128/JVI.06478-11
HIV cell-to-cell transmission requires the production of infectious virus particles and does not proceed through env-mediated fusion pores
Abstract
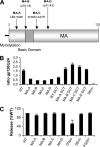
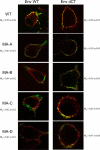
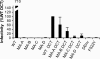
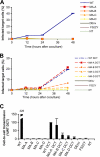
Direct cell-to-cell transmission of human immunodeficiency virus (HIV) is a more potent and efficient means of virus propagation than infection by cell-free virus particles. The aim of this study was to determine whether cell-to-cell transmission requires the assembly of enveloped virus particles or whether nucleic acids with replication potential could translocate directly from donor to target cells through envelope glycoprotein (Env)-induced fusion pores. To this end, we characterized the transmission properties of viruses carrying mutations in the matrix protein (MA) that affect the incorporation of Env into virus particles but do not interfere with Env-mediated cell-cell fusion. By use of cell-free virus, the infectivity of MA mutant viruses was below the detection threshold both in single-cycle and in multiple-cycle assays. Truncation of the cytoplasmic tail (CT) of Env restored the incorporation of Env into MA mutant viruses and rescued their cell-free infectivity to different extents. In cell-to-cell transmission assays, MA mutations prevented HIV transmission from donor to target cells, despite efficient Env-dependent membrane fusion. HIV transmission was blocked at the level of virus core translocation into the cytosol of target cells. As in cell-free assays, rescue of Env incorporation by truncation of the Env CT restored the virus core translocation and cell-to-cell infectivity of MA mutant viruses. These data show that HIV cell-to-cell transmission requires the assembly of enveloped virus particles. The increased efficiency of this infection route may thus be attributed to the high local concentrations of virus particles at sites of cellular contacts rather than to a qualitatively different transmission process.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

