Combination of biological screening in a cellular model of viral latency and virtual screening identifies novel compounds that reactivate HIV-1
- PMID: 22258251
- PMCID: PMC3302487
- DOI: 10.1128/JVI.05972-11
Combination of biological screening in a cellular model of viral latency and virtual screening identifies novel compounds that reactivate HIV-1
Abstract
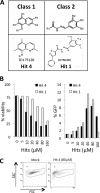
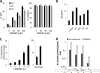
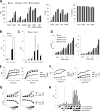
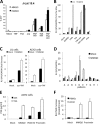
Although highly active antiretroviral therapy (HAART) has converted HIV into a chronic disease, a reservoir of HIV latently infected resting T cells prevents the eradication of the virus from patients. To achieve eradication, HAART must be combined with drugs that reactivate the dormant viruses. We examined this problem in an established model of HIV postintegration latency by screening a library of small molecules. Initially, we identified eight molecules that reactivated latent HIV. Using them as templates, additional hits were identified by means of similarity-based virtual screening. One of those hits, 8-methoxy-6-methylquinolin-4-ol (MMQO), proved to be useful to reactivate HIV-1 in different cellular models, especially in combination with other known reactivating agents, without causing T-cell activation and with lower toxicity than that of the initial hits. Interestingly, we have established that MMQO produces Jun N-terminal protein kinase (JNK) activation and enhances the T-cell receptor (TCR)/CD3 stimulation of HIV-1 reactivation from latency but inhibits CD3-induced interleukin-2 (IL-2) and tumor necrosis factor alpha (TNF-α) gene transcription. Moreover, MMQO prevents TCR-induced cell cycle progression and proliferation in primary T cells. The present study documents that the combination of biological screening in a cellular model of viral latency with virtual screening is useful for the identification of novel agents able to reactivate HIV-1. Moreover, we set the bases for a hypothetical therapy to reactivate latent HIV by combining MMQO with physiological or pharmacological TCR/CD3 stimulation.
Figures

Similar articles
-
A New Quinoline BRD4 Inhibitor Targets a Distinct Latent HIV-1 Reservoir for Reactivation from Other "Shock" Drugs.J Virol. 2018 Apr 27;92(10):e02056-17. doi: 10.1128/JVI.02056-17. Print 2018 May 15. J Virol. 2018. PMID: 29343578 Free PMC article.
-
Small-molecule screening using a human primary cell model of HIV latency identifies compounds that reverse latency without cellular activation.J Clin Invest. 2009 Nov;119(11):3473-86. doi: 10.1172/JCI39199. Epub 2009 Oct 1. J Clin Invest. 2009. PMID: 19805909 Free PMC article.
-
Novel structurally related compounds reactivate latent HIV-1 in a bcl-2-transduced primary CD4+ T cell model without inducing global T cell activation.J Antimicrob Chemother. 2012 Feb;67(2):398-403. doi: 10.1093/jac/dkr496. Epub 2011 Dec 7. J Antimicrob Chemother. 2012. PMID: 22160146 Free PMC article.
-
The role of latency reversal agents in the cure of HIV: A review of current data.Immunol Lett. 2018 Apr;196:135-139. doi: 10.1016/j.imlet.2018.02.004. Epub 2018 Feb 7. Immunol Lett. 2018. PMID: 29427743 Review.
-
Therapeutic Approaches to Eradicate Latent HIV-1 in Resting CD4+ T Cells.Curr Top Med Chem. 2016;16(10):1191-7. doi: 10.2174/1568026615666150901114138. Curr Top Med Chem. 2016. PMID: 26324046 Review.
Cited by
-
Mechanisms of HIV Transcriptional Regulation and Their Contribution to Latency.Mol Biol Int. 2012;2012:614120. doi: 10.1155/2012/614120. Epub 2012 Jun 3. Mol Biol Int. 2012. PMID: 22701796 Free PMC article.
-
Diversity of small molecule HIV-1 latency reversing agents identified in low- and high-throughput small molecule screens.Med Res Rev. 2020 May;40(3):881-908. doi: 10.1002/med.21638. Epub 2019 Oct 13. Med Res Rev. 2020. PMID: 31608481 Free PMC article. Review.
-
Triterpenoids from Ocimum labiatum Activates Latent HIV-1 Expression In Vitro: Potential for Use in Adjuvant Therapy.Molecules. 2017 Oct 13;22(10):1703. doi: 10.3390/molecules22101703. Molecules. 2017. PMID: 29027985 Free PMC article.
-
Impact of Myeloid Reservoirs in HIV Cure Trials.Curr HIV/AIDS Rep. 2019 Apr;16(2):129-140. doi: 10.1007/s11904-019-00438-5. Curr HIV/AIDS Rep. 2019. PMID: 30835045 Free PMC article. Review.
-
Histone deacetylase inhibitors (HDACis) that release the positive transcription elongation factor b (P-TEFb) from its inhibitory complex also activate HIV transcription.J Biol Chem. 2013 May 17;288(20):14400-14407. doi: 10.1074/jbc.M113.464834. Epub 2013 Mar 28. J Biol Chem. 2013. PMID: 23539624 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

