Enterovirus 71 disrupts interferon signaling by reducing the level of interferon receptor 1
- PMID: 22258259
- PMCID: PMC3302529
- DOI: 10.1128/JVI.06687-11
Enterovirus 71 disrupts interferon signaling by reducing the level of interferon receptor 1
Abstract
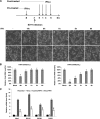
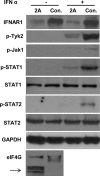
The recent outbreak of enterovirus 71 (EV71) infected millions of children and caused over 1,000 deaths. To date, neither an effective vaccine nor antiviral treatment is available for EV71 infection. Interferons (IFNs) have been successfully applied to treat patients with hepatitis B and C viral infections for decades but have failed to treat EV71 infections. Here, we provide the evidence that EV71 antagonizes type I IFN signaling by reducing the level of interferon receptor 1 (IFNAR1). We show that the host cells could sense EV71 infection and stimulate IFN-β production. However, the induction of downstream IFN-stimulated genes is inhibited by EV71. Also, only a slight interferon response and antiviral effects could be detected in cells treated with recombinant type I IFNs after EV71 infection. Further studies reveal that EV71 blocks the IFN-mediated phosphorylation of STAT1, STAT2, Jak1, and Tyk2 by reducing IFNAR1. Finally, we identified the 2A protease encoded by EV71 as an antagonist of IFNs and show that the protease activity is required for reducing IFNAR1 levels. Taken together, our study for the first time uncovers a mechanism used by EV71 to antagonize type I IFN signaling and provides new targets for future antiviral strategies.
Figures
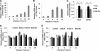


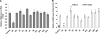

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

