Oligothiophenes as fluorescent markers for biological applications
- PMID: 22258339
- PMCID: PMC6268780
- DOI: 10.3390/molecules17010910
Oligothiophenes as fluorescent markers for biological applications
Abstract
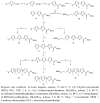
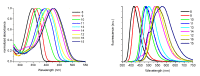
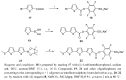
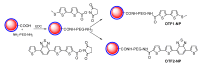
This paper summarizes some of our results on the application of oligothiophenes as fluorescent markers for biological studies. The oligomers of thiophene, widely known for their semiconductor properties in organic electronics, are also fluorescent compounds characterized by chemical and optical stability, high absorbance and quantum yield. Their fluorescent emission can be easily modulated via organic synthesis by changing the number of thiophene rings and the nature of side-chains. This review shows how oligothiophenes can be derivatized with active groups such as phosphoramidite, N-hydroxysuccinimidyl and 4-sulfotetrafluorophenyl esters, isothiocyanate and azide by which the (bio)molecules of interest can be covalently bound. This paper also describes how molecules such as oligonucleotides, proteins and even nanoparticles, tagged with oligothiophenes, can be used in experiments ranging from hybridization studies to imaging of fixed and living cells. Finally, a few multilabeling experiments are described.
Figures















References
-
- Golgi C. Sulla struttura della sostanza grigia dell cervello. Gazz. Med. Lombarda. 1873;33:244–246.
-
- Gram H.C. Uber die isolierte Färbung der Schizomyceten in Schnitt und Trockenpräparaten. Fortschritte der Medizin. 1884;2:185–189.
-
- Giemsa G. Eine Vereinfachung und Vervollkommnung meiner Methylenblau-Eosin-Färbemethode zur Erzielung der Romanowsky-Nocht’schen Chromatinfärbung. Centralblatt für Bakteriologie I Abteilung. 1904;32:207–313.
-
- Wijaya A., Schaffer S.B., Pallares I.G., Hamad-Schifferli K. Selective release of multiple DNA oligonucleotides from gold nanorods. Nano. 2009;3:80–86. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

