Antioxidant effect of Stryphnodendron rotundifolium Martius extracts from Cariri-Ceará State (Brazil): potential involvement in its therapeutic use
- PMID: 22258340
- PMCID: PMC6268277
- DOI: 10.3390/molecules17010934
Antioxidant effect of Stryphnodendron rotundifolium Martius extracts from Cariri-Ceará State (Brazil): potential involvement in its therapeutic use
Abstract
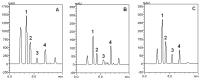
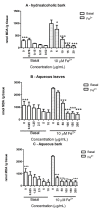
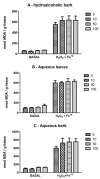
Stryphnodendron rotundifolium is a phytotherapic used in the northeast of Brazil for the treatment of inflammatory processes which normally are associated with oxidative stress. Consequently, we have tested the antioxidant properties of hydroalcoholic (HAB) and aqueous extracts (AB) from the bark and aqueous extract (AL) from the leaves of Stryphnodendron rotundifolium to determine a possible association between antioxidant activity and the popular use of this plant. Free radical scavenger properties were assessed by the quenching of 1',1'-diphenil-2-picrylhydrazyl (DPPH) and the calculated IC(50) were: HAB = 5.4 ± 0.7, AB = 12.0 ± 2.6, and AL = 46.3 ± 12.3 µg/mL. Total phenolic contents were: HAB = 102.7 ± 2.8, AB = 114.4 ± 14.6, and AL = 93.8 ± 9.1 µg/mg plant). HPLC/DAD analyses indicated that gallic acid, catechin, rutin and caffeic acid were the major components of the crude extracts of S. rotundifolium. Plant extracts inhibited Fe(II)-induced lipid peroxidation in brain homogenates. Iron chelation was also investigated and only HBA exhibited a weak activity. Taken together, the results suggest that S. rotundifolium could be considered an effective agent in the prevention of diseases associated with oxidative stress.
Figures


References
-
- Halliwell B. Free radicals, antioxidants and human diseases: Curiosity, cause or consequences. Lancet. 1994;334:721–724. - PubMed
-
- Jomova K., Jenisova Z., Feszterova M., Baros S., Liska J., Hudecova D., Rhodes C.J., Valko M. Arsenic: Toxicity, oxidative stress and human disease. J. Appl. Toxicol. 2011;31:95–107. - PubMed
-
- Jomova K., Vondrakova D., Lawson M., Valko M. Metals, oxidative stress and neurodegenerative disorders. Mol. Cell. Biochem. 2010;345:91–104. - PubMed
-
- Giorgio M., Trinei M., Migliaccio E., Pier G.P. Hydrogen peroxide: A metabolic by-product or a common mediator of ageing signals? Nat. Rev. Mol. Cell Biol. 2007;9:722–728. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

