ERK2 phosphorylation of serine 77 regulates Bmf pro-apoptotic activity
- PMID: 22258404
- PMCID: PMC3270271
- DOI: 10.1038/cddis.2011.137
ERK2 phosphorylation of serine 77 regulates Bmf pro-apoptotic activity
Abstract
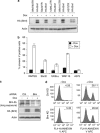
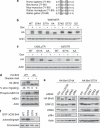
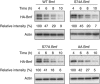
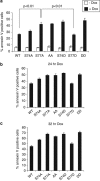
B-cell lymphoma 2 (Bcl-2) homology 3 (BH3)-only proteins represent a class of pro-apoptotic factors that neutralize pro-survival Bcl-2 proteins, and, in some cases, directly activate Bax. The mechanisms of control and the role of BH3-only proteins, such as Bcl-2 like protein 11 extra large and Bad are well studied. By contrast, relatively little is known about the regulation and role of Bcl-2 modifying factor (Bmf). The B-RAF oncogene is mutated in ∼8% of human tumors. We have previously shown that Bmf is upregulated at the transcript level and is required for apoptosis induced by targeting B-RAF signaling in tumor cells harboring mutant B-RAF. In this study, we show that Bmf is regulated at the post-translational level by mutant B-RAF-MEK-ERK2 signaling. Extracellular signal-regulated kinase (ERK2) directly phosphorylates Bmf on serine 74 and serine 77 residues with serine 77 being the predominant site. In addition, serine 77 phosphorylation reduces Bmf pro-apoptotic activity likely through a mechanism independent of altering Bmf localization to the mitochondria and/or interactions with dynein light chain 2 and the pro-survival proteins, B-cell lymphoma extra large, Bcl-2 and Mcl-1. These data identify a novel mode of regulation in Bmf that modulates its pro-apoptotic activity in mutant B-RAF tumor cells.
Figures

References
-
- Certo M, Del Gaizo Moore V, Nishino M, Wei G, Korsmeyer S, Armstrong SA, et al. Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell. 2006;9:351–365. - PubMed
-
- Cheng EH, Wei MC, Weiler S, Flavell RA, Mak TW, Lindsten T, et al. BCL-2, BCL-X(L) sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. Mol Cell. 2001;8:705–711. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

