Selective tumor killing based on specific DNA-damage response deficiencies
- PMID: 22258411
- PMCID: PMC3367714
- DOI: 10.4161/cbt.18921
Selective tumor killing based on specific DNA-damage response deficiencies
Abstract
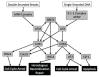
Organisms constantly undergo various stresses within their life span, which can damage their DNA. In order to maintain genomic stability and counteract the development of unwanted genomic mutations, organisms have evolved a DNA-damage response (DDR) to protect their genome. Due to the critical roles played by DDR in genomic stability, its defects can lead to cellular transformation and potentially tumorigenesis. Consequently, this also provides the opportunity to specifically target tumor cells due to a weakened ability to tolerate genotoxic stresses. In this lies a treatment strategy in which the inhibition of remaining DDR pathways can hyper-sensitize tumors to chemotherapeutic agents while minimizing deleterious effects to healthy cells. Therefore it is important to understand the genotypic background of specific tumors to determine which DDR pathways remain and can be targeted for inhibition. Tumor therapies based on the DDR are ideal not only as a means of increasing the effectiveness of current chemotherapies but also as a means to selectively target tumor cells while leaving healthy cells unharmed. Thus, targeting DDR components as a means of increasing effectiveness and discrimination of current chemotherapeutic tumor treatments is currently the focus of many studies and clinical trials.
Figures
Similar articles
-
Analysis of DNA Damage Response Gene Alterations and Tumor Mutational Burden Across 17,486 Tubular Gastrointestinal Carcinomas: Implications for Therapy.Oncologist. 2019 Oct;24(10):1340-1347. doi: 10.1634/theoncologist.2019-0034. Epub 2019 Apr 30. Oncologist. 2019. PMID: 31040255 Free PMC article.
-
DNA damage response pathways in tumor suppression and cancer treatment.World J Surg. 2009 Apr;33(4):661-6. doi: 10.1007/s00268-008-9840-1. World J Surg. 2009. PMID: 19034564 Review.
-
Noncoding RNAs in DNA Damage Response: Opportunities for Cancer Therapeutics.Methods Mol Biol. 2018;1699:3-21. doi: 10.1007/978-1-4939-7435-1_1. Methods Mol Biol. 2018. PMID: 29086365
-
The Adenovirus E4orf4 Protein Provides a Novel Mechanism for Inhibition of the DNA Damage Response.PLoS Pathog. 2016 Feb 11;12(2):e1005420. doi: 10.1371/journal.ppat.1005420. eCollection 2016 Feb. PLoS Pathog. 2016. PMID: 26867009 Free PMC article.
-
MicroRNAs, DNA Damage Response, and Cancer Treatment.Int J Mol Sci. 2016 Dec 12;17(12):2087. doi: 10.3390/ijms17122087. Int J Mol Sci. 2016. PMID: 27973455 Free PMC article. Review.
Cited by
-
Trial Watch: Targeting ATM-CHK2 and ATR-CHK1 pathways for anticancer therapy.Mol Cell Oncol. 2015 Feb 23;2(4):e1012976. doi: 10.1080/23723556.2015.1012976. eCollection 2015 Oct-Dec. Mol Cell Oncol. 2015. PMID: 27308506 Free PMC article. Review.
-
The transcription factor Cux1 in cerebellar granule cell development and medulloblastoma pathogenesis.Cerebellum. 2014 Dec;13(6):698-712. doi: 10.1007/s12311-014-0588-x. Cerebellum. 2014. PMID: 25096634
-
Rare Genetic Diseases with Defects in DNA Repair: Opportunities and Challenges in Orphan Drug Development for Targeted Cancer Therapy.Cancers (Basel). 2018 Sep 1;10(9):298. doi: 10.3390/cancers10090298. Cancers (Basel). 2018. PMID: 30200453 Free PMC article. Review.
-
E1B and E4 oncoproteins of adenovirus antagonize the effect of apoptosis inducing factor.Virology. 2014 May;456-457:205-19. doi: 10.1016/j.virol.2014.03.010. Epub 2014 Apr 15. Virology. 2014. PMID: 24889240 Free PMC article.
-
Targeting MUS81 promotes the anticancer effect of WEE1 inhibitor and immune checkpoint blocking combination therapy via activating cGAS/STING signaling in gastric cancer cells.J Exp Clin Cancer Res. 2021 Oct 8;40(1):315. doi: 10.1186/s13046-021-02120-4. J Exp Clin Cancer Res. 2021. PMID: 34625086 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
