Population pharmacokinetics and pharmacodynamics of piperaquine in children with uncomplicated falciparum malaria
- PMID: 22258469
- PMCID: PMC3736305
- DOI: 10.1038/clpt.2011.254
Population pharmacokinetics and pharmacodynamics of piperaquine in children with uncomplicated falciparum malaria
Abstract
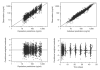
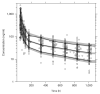
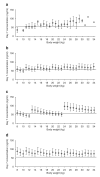
Dihydroartemisinin-piperaquine is being increasingly used as a first-line artemisinin combination treatment for malaria. The aim of this study was to describe the pharmacokinetic and pharmacodynamic properties of piperaquine in 236 children with uncomplicated falciparum malaria in Burkina Faso. They received a standard body weight-based oral 3-day fixed-dose dihydroartemisinin-piperaquine regimen. Capillary plasma concentration-time profiles were characterized using nonlinear mixed-effects modeling. The population pharmacokinetics of piperaquine were described accurately by a two-transit-compartment absorption model and a three-compartment distribution model. Body weight was a significant covariate affecting clearance and volume parameters. The individually predicted day 7 capillary plasma concentration of piperaquine was an important predictor (P < 0.0001) of recurrent malaria infection after treatment. Young children (2-5 years of age) received a significantly higher body weight-normalized dose than older children (P = 0.025) but had significantly lower day 7 piperaquine concentrations (P = 0.024) and total piperaquine exposures (P = 0.021), suggesting that an increased dose regimen for young children should be evaluated.
Figures

References
-
- Pashynska VA, Van den heuvel H, Claeys M, Kosevich MV. Characterization of noncovalent complexes of antimalarial agents of the artemisinin-type and FE(III)-heme by electrospray mass spectrometry and collisional activation tandem mass spectrometry. J. Am. Soc. Mass Spectrom. 2004;15:1181–1190. - PubMed
-
- World Health Organization Guidelines for the treatment of malaria. 2010.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

