Steroids for acute spinal cord injury
- PMID: 22258943
- PMCID: PMC6513405
- DOI: 10.1002/14651858.CD001046.pub2
Steroids for acute spinal cord injury
Abstract
Background: Acute spinal cord injury is a devastating condition typically affecting young people, mostly males. Steroid treatment in the early hours after the injury is aimed at reducing the extent of permanent paralysis during the rest of the patient's life.
Objectives: To review randomized trials of steroids for human acute spinal cord injury.
Search methods: We searched the Cochrane Injuries Group Specialised Register (searched 02 Aug 2011), The Cochrane Central Register of Controlled Trials 2011, issue 3 (The Cochrane Library), MEDLINE (Ovid) 1948 to July Week 3 2011, EMBASE (Ovid) 1974 to 2011 week 17, ISI Web of Science: Science Citation Index Expanded (SCI-EXPANDED) 1970 to Aug 2011, ISI Web of Science: Conference Proceedings Citation Index- Science (CPCI-S) 1990 to Aug 2011 and PubMed [www.ncbi.nlm.nih.gov/sites/entrez/] (searched 04 Aug 2011) for records added to PubMed in the last 90 days). Files of the National Acute Spinal Cord Injury Study (NASCIS) were reviewed (NASCIS was founded in 1977 and has tracked trials in this area since that date). We also searched the reference lists of relevant studies and previously published reviews.
Selection criteria: All randomized controlled trials of steroid treatment for acute spinal cord injury in any language.
Data collection and analysis: One review author extracted data from trial reports. Japanese and French studies were found through NASCIS and additional data (e.g. SDs) were obtained from the original study authors.
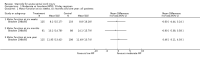
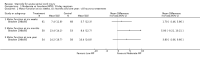
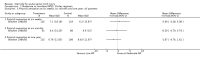
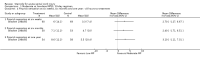
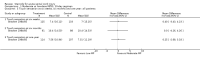
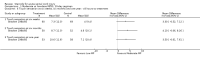
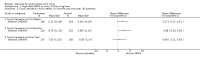
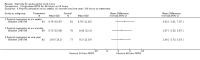
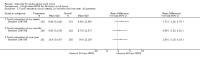
Main results: Eight trials are included in this review, seven used methylprednisolone. Methylprednisolone sodium succinate has been shown to improve neurologic outcome up to one year post-injury if administered within eight hours of injury and in a dose regimen of: bolus 30mg/kg over 15 minutes, with maintenance infusion of 5.4 mg/kg per hour infused for 23 hours. The initial North American trial results were replicated in a Japanese trial but not in the one from France. Data was obtained from the latter studies to permit appropriate meta-analysis of all three trials. This indicated significant recovery in motor function after methylprednisolone therapy, when administration commenced within eight hours of injury. A more recent trial indicates that, if methylprednisolone therapy is given for an additional 24 hours (a total of 48 hours), additional improvement in motor neurologic function and functional status are observed. This is particularly observed if treatment cannot be started until between three to eight hours after injury. The same methylprednisolone therapy has been found effective in whiplash injuries. A modified regimen was found to improve recovery after surgery for lumbar disc disease. The risk of bias was low in the largest methyprednisolne trials. Overall, there was no evidence of significantly increased complications or mortality from the 23 or 48 hour therapy.
Authors' conclusions: High-dose methylprednisolone steroid therapy is the only pharmacologic therapy shown to have efficacy in a phase three randomized trial when administered within eight hours of injury. One trial indicates additional benefit by extending the maintenance dose from 24 to 48 hours, if start of treatment must be delayed to between three and eight hours after injury. There is an urgent need for more randomized trials of pharmacologic therapy for acute spinal cord injury.
Conflict of interest statement
Professor Bracken was an occasional consultant to Pharmacia & Upjohn Inc and is an author on several of the papers included in this review.
Figures




















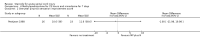
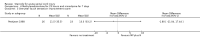
Update of
-
Steroids for acute spinal cord injury.Cochrane Database Syst Rev. 2002;(3):CD001046. doi: 10.1002/14651858.CD001046. Cochrane Database Syst Rev. 2002. Update in: Cochrane Database Syst Rev. 2012 Jan 18;1:CD001046. doi: 10.1002/14651858.CD001046.pub2. PMID: 12137616 Updated.
References
References to studies included in this review
Bracken 1984/85 {published data only}
-
- Bracken MB, Collins WF, Freeman DF, Shepard MJ, Wagner FW, Silten RM, et al. Efficacy of methylprednisolone in acute spinal cord injury. Journal of the American Medical Association 1984;251:45‐52. - PubMed
-
- Bracken MB, Shepard MJ, Hellenbrand KG, Collins WF, Leo LS, Freeman DF, et al. Methylprednisolone and neurological function one year after spinal cord injury. Journal of Neurosurgery 1985;63:704‐13. - PubMed
Bracken 1990/93 {published data only}
-
- Bracken MB. Pharmacological treatment of acute spinal cord injury: current status and future prospects. Paraplegia 1992;30:102‐7. - PubMed
-
- Bracken MB, Holford TR. Effects of timing of methylprednisolone or naloxone on recovery of segmental and long‐tract neurological function in NASCIS 2. Journal of Neurosurgery 1993;79:500‐7. - PubMed
-
- Bracken MB, Shepard MJ, Collins WF, et al. Methylprednisolone or naloxone treatment after acute spinal cord injury: 1 year follow‐up data. Journal of Neurosurgery 1992;76:23‐31. - PubMed
-
- Bracken MB, Shepard MJ, Collins WF, Holford TR, Young W, Baskin DS, et al. A randomized controlled trial of methylprednisolone or naloxone in the treatment of acute spinal cord injury. New England Journal of Medicine 1990;322:1405‐11. - PubMed
-
- Duh M‐S, Shepard MJ, Wilberger JE, Bracken MB. The effectiveness of surgery on the treatment of acute spinal cord injury and its relation to pharmacological treatment. Neurosurgery 1994;35:240‐9. - PubMed
Bracken 1997/98 {published data only}
-
- Bracken MB, Shepard MJ, Holford TR, Leo‐Summers L, Aldrich EF, Fazl M, et al. Administration of methylprednisolone for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment of acute spinal cord injury. Results of the third national acute spinal cord injury randomized controlled trial. Journal of the American Medical Association 1997;277:1597‐604. - PubMed
-
- Bracken MB, Shepard MJ, Holford TR, Leo‐Summers L, Aldrich EF, Fazl M, et al. Methylprednisolone or tirilazad mesylate administration after acute spinal cord injury: 1‐year follow up. Results of the third National Acute Spinal Cord Injury randomized controlled trial. Journal of Neurosurgery 1998;89:699‐706. - PubMed
Glasser 1993 {published data only}
-
- Glasser RS, Knego RS, Delashaw JB, Fessler RG. The perioperative use of corticosteroids and bupivacaine in the management of lumbar disc disease. Journal of Neurosurgery 1993;78:383‐7. - PubMed
Matsumoto 2001 {published data only}
-
- Matsumoto T, Tamaki T, Kawakami M, Yoshida M, Ando M, Yamada H. Early complications of high dose methylprednisolone sodium succinate treatment in the follow‐up of acute cervical spinal cord injury. Spine 2001;26(4):426‐30. - PubMed
Otani 1994 {published data only}
-
- Otani K. Functional recovery: focus on upper and lower limbs (translation of Japanese). Journal of the Japanese Paraplegia Medicine Association 1995;8:80‐1.
-
- Otani K, Abe H, Kadoya S, et al. Beneficial effect of methylprednisolone sodium succinate in the treatment of acute spinal cord injury (translation of Japanese). Sekitsui Sekizui J 1994;7:633‐47.
-
- Yokota H, Kawai M, Kato K, et al. Significance of methylprednisolone therapy in acute spinal cord injury with special reference to short term follow‐up (translation of Japanese). Journal of the Japanese Association of Acute Medicine 1995;6:349‐54.
Petitjean 1998 {published data only}
-
- Petitjean ME, Pointillart V, Dixmerias F, Wiart L, Sztark F, Lassie P, Thicoipe M, Dabadie P. Traitement medicamenteux de la lesion medullaire traumatique au stade aigu. Annales françaises d'anesthèsie et de rèanimation 1998;17:115‐22. - PubMed
Pettersson 1998 {published data only}
-
- Pettersson K, Toolanen G. High dose methylprednisolone prevents extensive sick‐leave after whiplash injury. A prospective randomized double‐blind study. Spine 1998;23:984‐9. - PubMed
References to studies excluded from this review
Kiwerski 1992 {published data only}
-
- Kiwerski J. Zastosowanie dexametazonu w leczeniu uszkodzen rdzenia kregowego we wczesnym okresie pourazowym. Neur Neurochir Pol 1992;26:518‐27. - PubMed
Pointillart 2000 {published data only}
-
- Pointillart V, Petitjean ME, Vital JM, Lassie P, Thicoipe M, Dabadie P. Pharmacological therapy of spinal cord injury during the acute phase. Spinal Cord 2000;38:71‐6. - PubMed
Yokota 1995 {published data only}
-
- Yokota H, Kawai M, Kato K, Mashiko K, Yamamoto Y, Henmi H, Otsuka T. Significance of methylprednisolone therapy in acute spinal cord injury with special reference to short‐term follow‐up. Journal of the Japanese Association of Acute Medicine 1995;6:349‐54.
Additional references
Berkowitz 1992
-
- Berkowitz M, Harvey C, Greene CG, Wilson SE. The economic consequences of traumatic spinal cord injury. New York: Demos Publications, 1992.
Bracken 1981
-
- Bracken MB, Freeman DH, Hellenbrand K. Incidence of acute traumatic hospitalized spinal cord injury in the United States 1970‐1977. American Journal of Epidemiology 1981;133:615‐22. - PubMed
Bracken 2001
-
- Bracken MB. Methylprednisolone and acute spinal cord injury: an update of the randomized evidence. Spine 2001;26:S47‐55. - PubMed
Ducker 1969
-
- Ducker TB, Hamit HF. Experimental treatments of acute spinal cord injury. Journal of Neurosurgery 1969;30:693‐722. - PubMed
Follmann 1992
-
- Follmann D, Elliott P, Cutler J. Variance imputation for overviews of clinical trials with continuous response. Journal of Clinical Epidemiology 1992;45:769‐73. - PubMed
Hall 1992
-
- Hall ED. The neuroprotective pharmacology of methylprednisolone. Journal of Neurosurgery 1992;76:13‐22. - PubMed
Sauerland 2000
-
- Sauerland S, Nagelschmidt M, Mallman P, et al. Risks and benefits of preoperative high dose methylprednisolone in surgical patients: a systematc review. Drug Safety 2000;55:452‐3. - PubMed
Sinclair 1992
-
- Sinclair JC, Bracken MB (eds). Effective care of the newborn infant. Oxford: Oxford University Press, 1992:p9.
Wing 1998
-
- Wing PC, Nance P, Connell DG, et al. Risk of avascular necrosis following short term megadose methylprednisolone treatment. Spinal Cord 1998;36:633‐6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

