Bezafibrate for primary biliary cirrhosis
- PMID: 22259000
- PMCID: PMC11338546
- DOI: 10.1002/14651858.CD009145.pub2
Bezafibrate for primary biliary cirrhosis
Abstract
Background: Treatment of primary biliary cirrhosis is complicated. There are studies suggesting that bezafibrate, alone or in combination with ursodeoxycholic acid (UDCA), is effective in the treatment of primary biliary cirrhosis, but no systematic review has summarised the evidence yet.
Objectives: To assess the beneficial and harmful effects of bezafibrate in patients with primary biliary cirrhosis.
Search methods: The Cochrane Hepato-Biliary Group Controlled Trials Register, The Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library, MEDLINE, EMBASE, Science Citation Index Expanded, LILACS, Clinicaltrials.gov, the WHO International Clinical Trials Registry Platform, and full text searches were conducted until November 2011. The searches in Chinese Bio-medical Literature Database, China Network Knowledge Information, Chinese Science Journal Database, Chinese Medical Citation Index, Wanfang Database, and full text searches were conducted until January 2011. Manufacturers and authors were contacted.
Selection criteria: All randomised clinical trials comparing bezafibrate at any dose or regimen in patients with primary biliary cirrhosis with placebo or no intervention, or with another drug. Any concomitant interventions were allowed if received equally by all treatment groups in a trial.
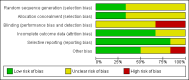
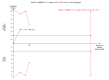
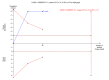
Data collection and analysis: Two authors extracted data. RevMan Analysis was used for statistical analysis of dichotomous data with risk ratio (RR) or risk difference (RD), and of continuous data with mean difference (MD), both with 95% confidence intervals (CI). Methodological domains were used to assess risk of systematic errors (bias). Trial sequential analysis was used to control for random errors (play of chance).
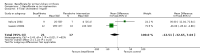
Main results: Six trials with 151 Japanese patients were included. All trials had high risk of bias. Four trials compared bezafibrate plus UDCA with no intervention plus UDCA (referenced as bezafibrate versus no intervention in the remaining text), and two trials compared bezafibrate with UDCA. No patient died and no patient developed liver-related complications in any of the included trials. Bezafibrate was without significant effects on the occurrence of adverse events compared with no intervention (5/32 (16%) versus 0/28 (0%)) (RR 5.40, 95% CI 0.69 to 42.32; 3 trials with 60 patients; I² = 0%) or with UDCA (2/32 (6%) versus 0/37 (0%)) (RR 6.19, 95% CI 0.31 to 122.05; 2 trials with 69 patients; I² = 0%). Bezafibrate significantly decreased the activity of serum alkaline phosphatases compared with no intervention (MD -186.04 U/L, 95% CI -249.03 to -123.04; 4 trials with 79 patients; I² = 34%) and when compared with UDCA (MD -162.90 U/L, 95% CI -199.68 to -126.12; 2 trials with 48 patients; I² = 0%). These results were supported by trial sequential analyses. Bezafibrate compared with no intervention significantly decreased plasma immunoglobulin M (MD -164.00 mg/dl, 95% CI -259.47 to -68.53; 3 trials with 50 patients; I² = 46%) and serum bilirubin concentration (MD -0.19 mg/dl, 95% CI -0.38 to -0.00; 2 trials with 34 patients; I² = 0%). However, the latter two results were not supported by trial sequential analyses. Bezafibrate compared with no intervention had no significant effect on the activity of serum gamma-glutamyltransferase (MD -1.22 U/L, 95% CI -11.97 to 9.52; 4 trials with 79 patients; I² = 42%) and serum alanine aminotransferase (MD -5.61 U/L, 95% CI -24.50 to 13.27; 2 trials with 35 patients; I² = 34%). Bezafibrate compared with UDCA had no significant effect on the activity of serum gamma-glutamyltransferase (MD 38.44 U/L, 95% CI -180.67 to 257.55; 2 trials with 49 patients; I² = 89%), serum alanine aminotransferase (MD -2.34 U/L, 95% CI -34.73 to 30.06; 2 trials with 49 patients; I² = 95%), and plasma immunoglobulin M concentration (MD -20.23 mg/dl, 95% CI -218.71 to 178.25; 2 trials with 41 patients; I² = 90%) in random-effects model meta-analyses, but bezafibrate significantly decreased the activity of serum gamma-glutamyltransferase (MD -58.18, 95% CI -76.49 to -39.88; 2 trials with 49 patients; I² = 89%), serum alanine aminotransferase (MD -13.94, 95% CI -18.78 to -9.09; 2 trials with 49 patients; I² = 95%), and plasma immunoglobulin M concentration (MD -99.90, 95% CI -130.72 to -69.07; 2 trials with 41 patients; I² = 90%) in fixed-effect model meta-analyses. One patient had bezafibrate withdrawn due to an adverse event compared to no intervention (RD 0.03, 95% CI -0.09 to 0.16; 2 trials with 60 patients; I² = 0%).
Authors' conclusions: This systematic review did not demonstrate any effect of bezafibrate versus no intervention on mortality, liver-related morbidity, adverse events, and pruritus in patients with primary biliary cirrhosis. Furthermore, we found no significant effects of bezafibrate on mortality, liver-related morbidity, or adverse events when compared with ursodeoxycholic acid, None of the trials assessed quality of life or fatigue. The data seem to indicate a possible positive intervention effect of bezafibrate on some liver biochemistry measures compared with the control group, but the observed effects could be due to systematic errors or random errors. We need more randomised clinical trials on the effects of bezafibrate on primary biliary cirrhosis with low risks of systematic errors and random errors.
Conflict of interest statement
None known.
Figures























Update of
- doi: 10.1002/14651858.CD009145
References
References to studies included in this review
Itakura 2004 {published data only}
-
- Itakura J, Izumi N, Nishimura Y, Inoue K, Ueda K, Nakanishi H, et al. Prospective randomized crossover trial of combination therapy with bezafibrate and UDCA for primary biliary cirrhosis. Hepatology Research 2004;29(4):216‐22. - PubMed
Iwasaki 2008a {published data only}
-
- Iwasaki S, Ohira H, Nishiguchi S, Zeniya M, Kaneko S, Onji M, et al. The efficacy of ursodeoxycholic acid and bezafibrate combination therapy for primary biliary cirrhosis: a prospective, multicenter study. Hepatology Research 2008;38(6):557‐64. - PubMed
Iwasaki 2008b {published data only}
-
- Iwasaki S, Ohira H, Nishiguchi S, Zeniya M, Kaneko S, Onji M, et al. The efficacy of ursodeoxycholic acid and bezafibrate combination therapy for primary biliary cirrhosis: a prospective, multicenter study. Hepatology Research 2008;38(6):557‐64. - PubMed
Kanda 2003 {published data only}
-
- Kanda T, Yokosuka O, Imazeki F, Saisho H. Bezafibrate treatment: a new medical approach for PBC patients?. Journal of Gastroenterology 2003;38(6):573‐8. - PubMed
Kurihara 2000 {published data only}
-
- Kurihara T, Niimi A, Maeda A, Shigemoto M, Yamashita K. Bezafibrate in the treatment of primary biliary cirrhosis: comparison with ursodeoxycholic acid. American Journal of Gastroenterology 2000; Vol. 95, issue 10:2990‐2. - PubMed
Nakai 1999 {published data only}
-
- Nakai S, Masaki T, Kurokohchi K, Deguchi A, Nishioka M. Combination therapy of bezafibrate and ursodeoxycholic acid in primary biliary cirrhosis: a preliminary study. American Journal of Gastroenterology 2000;95(1):326‐7. - PubMed
-
- Nakai S, Masaki T, Morita T, Deguchi A, Nishioka M. The effect of bezafibrate in patients with primary biliary cirrhosis [abstract]. Hepatology 1999; Vol. 30, issue 4:566A.
References to studies excluded from this review
Fukuo 1996 {published data only}
-
- Fukuo Y, Kitami T, Nomoto T, Terashi A. A lipid lowering drug (bezafibrate) has a favourable effect on liver enzymes (ALP and gamma‐GTP). Nippon Ika Daigaku Zasshi 1996;63(5):424‐30. - PubMed
Hazzan 2010 {published data only}
-
- Hazzan R, Tur‐Kaspa R. Bezafibrate treatment of primary biliary cirrhosis following incomplete response to ursodeoxycholic acid. Journal of Clinical Gastroenterology 2010;44(5):371‐3. - PubMed
Iwasaki 1999 {published data only}
-
- Iwasaki S, Tsuda K, Ueta H, Aono R, Ono M, Saibara T, et al. Bezafibrate may have a beneficial effect in pre‐cirrhotic primary biliary cirrhosis. Hepatology Research 1999;16:12‐8.
Miyaguchi 2000 {published data only}
-
- Miyaguchi S, Ebinuma H, Imaeda H, Nitta Y, Watanabe T, Saito H, et al. A novel treatment for refractory primary biliary cirrhosis?. Hepato‐Gastroenterology 2000;47(36):1518‐21. - PubMed
Ohmoto 2001 {published data only}
-
- Ohmoto K, Mitsui Y, Yamamoto S. Effect of bezafibrate in primary biliary cirrhosis: a pilot study. Liver 2001;21(3):223‐4. - PubMed
References to ongoing studies
JPRN‐C000000225 {published data only}
-
- Randomised clinical trial of ursodeoxycholic acid with or without bezafibrate in primary biliary cirrhosis.. Ongoing study December 2003..
Additional references
AASLD 2010
-
- Silveira MG, Brunt EM, Heathcote J, Gores GJ, Lindor KD, Mayo MJ. American Association for the Study of Liver Diseases endpoints conference: design and endpoints for clinical trials in primary biliary cirrhosis. Hepatology 2010;52(1):349‐59. - PubMed
Brok 2008
-
- Brok J, Thorlund K, Gluud C, Wetterslev J. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta‐analyses. Journal of Clinical Epidemiology 2008;61(8):763‐9. - PubMed
Chianale 1996
Delerive 2001
-
- Delerive P, Fruchart JC, Staels B. Peroxisome proliferator‐activated receptors in inflammation control. Journal of Endocrinology 2001;169(3):453‐9. - PubMed
DeMets 1987
-
- DeMets DL. Methods for combining randomized clinical trials: strengths and limitations. Statistics in Medicine 1987;6(3):341‐50. - PubMed
DerSimonian 1986
-
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. - PubMed
Devchand 1996
-
- Devchand PR, Keller H, Peters JM, Vazquez M, Gonzalez FJ, Wahli W. The PPARalpha‐leukotriene B4 pathway to inflammation control. Nature 1996;384(6604):39‐43. - PubMed
EASL 2009
-
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of cholestatic liver diseases. Journal of Hepatology 2009;51(2):237‐67. - PubMed
Egger 1997
Egger 2003
-
- Egger M, Juni P, Bartlett C, Holenstein F, Sterne J. How important are comprehensive literature searches and the assessment of trial quality in systematic reviews? Empirical study.. Health Technology Assessment 2003;7(1):1‐76. - PubMed
Gluud 2006
-
- Gluud LL. Bias in clinical intervention research. American Journal of Epidemiology 2006;163(6):493‐501. - PubMed
Gluud 2007
-
- Gluud C, Brok J, Gong Y, Koretz RL. Hepatology may have problems with putative surrogate outcome measures. Journal of Hepatology 2007;46(4):734‐42. - PubMed
Gluud 2011
-
- Gluud C, Nikolova D, Klingenberg SL, Alexakis N, Als‐Nielsen B, Colli A, et al. Cochrane Hepato‐Biliary Group. About The Cochrane Collaboration (Cochrane Review Groups (CRGs)). 2011, Issue 10. Art. No.: LIVER.
Heathcote 2000
-
- Heathcote EJ. Management of primary biliary cirrhosis. The American Association for the Study of Liver Diseases practice guidelines. Hepatology 2000;31(4):1005‐13. - PubMed
Higgins 2002
-
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. - PubMed
Higgins 2003
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hirano 2002
-
- Hirano Y, Hirano F, Fujii H, Makino I. Fibrates suppress chenodeoxycholic acid‐induced RANTES expression through inhibition of NF‐kappaB activation. European Journal of Pharmacology 2002;448(1):19‐26. - PubMed
Hollis 1999
ICH‐GCP 1997
-
- International Conference on Harmonisation Expert Working Group. International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use. ICH harmonised tripartite guideline. Guideline for good clinical practice1997 CFR & ICH Guidelines. Vol. 1, PA 19063‐2043, USA: Barnett International/PAREXEL, 1997.
Ishimaru 2002
-
- Ishimaru H, Iino S. Japanese abstract. Acta Hepatologica Japonica (Japanese Society of Hepatology) 2002;43(Suppl 2):A377.
Jacquemin 2001
-
- Jacquemin E, Vree JM, Cresteil D, Sokal EM, Sturm E, Dumont M, et al. The wide spectrum of multidrug resistance 3 deficiency: from neonatal cholestasis to cirrhosis of adulthood. Gastroenterology 2001;120(6):1448‐58. - PubMed
Kaplan 2005
-
- Kaplan MM, Gershwin ME. Primary biliary cirrhosis. New England Journal of Medicine 2005;353(12):1261‐73. - PubMed
Kjaergard 2001
-
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. - PubMed
Kurihara 2002
-
- Kurihara T, Maeda A, Shigemoto M, Yamashita K, Hashimoto E. Investigation into the efficacy of bezafibrate against primary biliary cirrhosis, with histological references from cases receiving long term monotherapy. American Journal of Gastroenterology 2002;97(1):212‐4. - PubMed
Lee 2005
-
- Lee YM, Kaplan MM. The natural history of PBC: has it changed?. Seminars in Liver Disease 2005;25(3):321‐6. - PubMed
Macaskill 2001
-
- Macaskill P, Walter SD, Irwig L. A comparison of methods to detect publication bias in meta‐analysis. Statistics in Medicine 2001;20(4):641‐54. - PubMed
Moher 1998
-
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352:609‐13. - PubMed
Muscari 2002
-
- Muscari A, Puddu GM, Puddu P. Lipid‐lowering drugs: are adverse effects predictable and reversible?. Cardiology 2002;97(3):115‐21. - PubMed
Nakai 2000
-
- Nakai S, Masaki T, Kurokohchi K, Deguchi A, Nishioka M. Combination therapy of bezafibrate and ursodeoxycholic acid in primary biliary cirrhosis: a preliminary study. American Journal of Gastroenterology 2000;95(1):326‐7. - PubMed
Poupon 2010
-
- Poupon R. Primary biliary cirrhosis: a 2010 update. Journal of Hepatology 2010;52(5):745‐58. - PubMed
Prince 2003
-
- Prince MI, James OF. The epidemiology of primary biliary cirrhosis. Clinics in Liver Diseases 2003;7(4):795‐819. - PubMed
RevMan 2011 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Ros 2003
-
- Ros JE, Libbrecht L, Geuken M, Jansen PL, Roskams TA. High expression of MDR1, MRP1, and MRP3 in the hepatic progenitor cell compartment and hepatocytes in severe human liver disease. Journal of Pathology 2003;200(5):553‐60. - PubMed
Royle 2003
-
- Royle P, Milne R. Literature searching for randomized controlled trials used in Cochrane reviews: rapid versus exhaustive searches. International Journal of Technology Assessment in Health Care 2003;19(4):591‐603. - PubMed
Schulz 1995
-
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. Journal of the American Medical Association 1995;273(5):408‐12. - PubMed
Shapiro 1979
Smit 1993
-
- Smit JJ, Schinkel AH, Oude Elferink RP, Groen AK, Wagenaar E, Deemter L, et al. Homozygous disruption of the murine mdr2 P‐glycoprotein gene leads to a complete absence of phospholipid from bile and to liver disease. Cell 1993;75(3):451‐62. - PubMed
Tenenbaum 2004
-
- Tenenbaum A, Motro M, Fisman EZ, Schwammenthal E, Adler Y, Goldenberg I, et al. Peroxisome proliferator‐activated receptor ligand bezafibrate for prevention of type 2 diabetes mellitus in patients with coronary artery disease. Circulation 2004;109(18):2197‐202. - PubMed
Tenenbaum 2005
-
- Tenenbaum A, Motro M, Fisman EZ, Tanne D, Boyko V, Behar S. Bezafibrate for the secondary prevention of myocardial infarction in patients with metabolic syndrome. Archives of Internal Medicine 2005;165(10):1154‐60. - PubMed
The BIP Study Group 2000
-
- The Bezafibrate Infarction Prevention (BIP) Study. Secondary prevention by raising HDL cholesterol and reducing triglycerides in patients with coronary artery disease. Circulation 2000;102(1):21‐7. - PubMed
Thorlund 2009
-
- Thorlund K, Devereaux PJ, Wetterslev J, Guyatt G, Ioannidis JP, Thabane L, et al. Can trial sequential monitoring boundaries reduce spurious inferences from meta‐analyses. International Journal of Epidemiology 2009;38(1):276‐86. - PubMed
Vessby 1980
-
- Vessby B, Lithell H, Hellsing K, Ostlund‐Lindqvist AM, Gustafsson IB, Boberg J, et al. Effects of bezafibrate on the serum lipoprotein lipid and apolipoprotein composition, lipoprotein triglyceride removal capacity and the fatty acid composition of the plasma lipid esters. Atherosclerosis 1980;37(2):257‐69. - PubMed
Wetterslev 2008
-
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta‐analysis. Journal of Clinical Epidemiology 2008;61(1):64‐75. - PubMed
Wetterslev 2009
Williams 1984
Wood 2008
Yano 2002
-
- Yano K, Kato H, Morita S, Takahara O, Ishibashi H, Furukawa R. Is bezafibrate histologically effective for primary biliary cirrhosis?. American Journal of Gastroenterology 2002;97(4):1075‐7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

