Activation of cyclic AMP signaling leads to different pathway alterations in lesions of the adrenal cortex caused by germline PRKAR1A defects versus those due to somatic GNAS mutations
- PMID: 22259056
- PMCID: PMC3319183
- DOI: 10.1210/jc.2011-3000
Activation of cyclic AMP signaling leads to different pathway alterations in lesions of the adrenal cortex caused by germline PRKAR1A defects versus those due to somatic GNAS mutations
Abstract
Context: The overwhelming majority of benign lesions of the adrenal cortex leading to Cushing syndrome are linked to one or another abnormality of the cAMP or protein kinase pathway. PRKAR1A-inactivating mutations are responsible for primary pigmented nodular adrenocortical disease, whereas somatic GNAS activating mutations cause macronodular disease in the context of McCune-Albright syndrome, ACTH-independent macronodular hyperplasia, and, rarely, cortisol-producing adenomas.
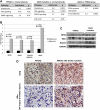
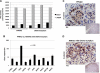
Objective and design: The whole-genome expression profile (WGEP) of normal (pooled) adrenals, PRKAR1A- (3) and GNAS-mutant (3) was studied. Quantitative RT-PCR and Western blot were used to validate WGEP findings.
Results: MAPK and p53 signaling pathways were highly overexpressed in all lesions against normal tissue. GNAS-mutant tissues were significantly enriched for extracellular matrix receptor interaction and focal adhesion pathways when compared with PRKAR1A-mutant (fold enrichment 3.5, P < 0.0001 and 2.1, P < 0.002, respectively). NFKB, NFKBIA, and TNFRSF1A were higher in GNAS-mutant tumors (P < 0.05). Genes related to the Wnt signaling pathway (CCND1, CTNNB1, LEF1, LRP5, WISP1, and WNT3) were overexpressed in PRKAR1A-mutant lesions.
Conclusion: WGEP analysis revealed that not all cAMP activation is the same: adrenal lesions harboring PRKAR1A or GNAS mutations share the downstream activation of certain oncogenic signals (such as MAPK and some cell cycle genes) but differ substantially in their effects on others.
Figures
References
-
- Bourdeau I, Matyakhina L, Stergiopoulos SG, Sandrini F, Boikos S, Stratakis CA. 2006. 17q22–24 chromosomal losses and alterations of protein kinase a subunit expression and activity in adrenocorticotropin-independent macronodular adrenal hyperplasia. J Clin Endocrinol Metab 91:3626–3632 - PubMed
-
- Bertherat J, Horvath A, Groussin L, Grabar S, Boikos S, Cazabat L, Libe R, René-Corail F, Stergiopoulos S, Bourdeau I, Bei T, Clauser E, Calender A, Kirschner LS, Bertagna X, Carney JA, Stratakis CA. 2009. Mutations in regulatory subunit type 1A of cyclic adenosine 5′-monophosphate-dependent protein kinase (PRKAR1A): phenotype analysis in 353 patients and 80 different genotypes. J Clin Endocrinol Metab 94:2085–2091 - PMC - PubMed
-
- Horvath A, Boikos S, Giatzakis C, Robinson-White A, Groussin L, Griffin KJ, Stein E, Levine E, Delimpasi G, Hsiao HP, Keil M, Heyerdahl S, Matyakhina L, Libé R, Fratticci A, Kirschner LS, Cramer K, Gaillard RC, Bertagna X, Carney JA, Bertherat J, Bossis I, Stratakis CA. 2006. A genome-wide scan identifies mutations in the gene encoding phosphodiesterase 11A4 (PDE11A) in individuals with adrenocortical hyperplasia. Nat Genet 38:794–800 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

