Krüppel-like factor 9 and progesterone receptor coregulation of decidualizing endometrial stromal cells: implications for the pathogenesis of endometriosis
- PMID: 22259059
- PMCID: PMC3319212
- DOI: 10.1210/jc.2011-2562
Krüppel-like factor 9 and progesterone receptor coregulation of decidualizing endometrial stromal cells: implications for the pathogenesis of endometriosis
Abstract
Context: Endometriosis is characterized by progesterone resistance and associated with infertility. Krüppel-like factor 9 (KLF9) is a progesterone receptor (PGR)-interacting protein, and mice null for Klf9 are subfertile. Whether loss of KLF9 expression contributes to progesterone resistance of eutopic endometrium of women with endometriosis is unknown.
Objective: The aims were to investigate 1) KLF9 expression in eutopic endometrium of women with and without endometriosis, 2) effects of attenuated KLF9 expression on WNT-signaling component expression and on WNT inhibitor Dickkopf-1 promoter activity in human endometrial stromal cells (HESC), and 3) PGR and KLF9 coregulation of the stromal transcriptome network.
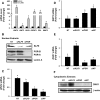
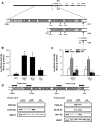
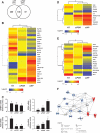
Methods: Transcript levels of KLF9, PGR, and WNT signaling components were measured in eutopic endometrium of women with and without endometriosis. Transcript and protein levels of WNT signaling components in HESC transfected with KLF9 and/or PGR small interfering RNA were analyzed by quantitative RT-PCR and Western blot. KLF9 and PGR coregulation of Dickkopf-1 promoter activity was evaluated using human Dickkopf-1-luciferase promoter/reporter constructs and by chromatin immunoprecipitation. KLF9 and PGR signaling networks were analyzed by gene expression array profiling.
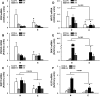
Results: Eutopic endometrium from women with endometriosis had reduced expression of KLF9 mRNA together with those of PGR-B, WNT4, WNT2, and DKK1. KLF9 and PGR were recruited to the DKK1 promoter and modified each other's transactivity. In HESC, KLF9 and PGR coregulated components of the WNT, cytokine, and IGF gene networks that are implicated in endometriosis and infertility.
Conclusion: Loss of KLF9 coregulation of endometrial stromal PGR-responsive gene networks may underlie progesterone resistance in endometriosis.
Figures
References
-
- Giudice LC, Kao LC. 2004. Endometriosis. Lancet 364:1789–1799 - PubMed
-
- Eskenazi B, Warner ML. 1997. Epidemiology of endometriosis. Obstet Gynecol Clin North Am 24:235–258 - PubMed
-
- Hahn DW, Carraher RP, Foldesy RG, McGuire JL. 1986. Experimental evidence for failure to implant as a mechanism of infertility associated with endometriosis. Am J Obstet Gynecol 155:1109–1113 - PubMed
-
- Illera MJ, Juan L, Stewart CL, Cullinan E, Ruman J, Lessey BA. 2000. Effect of peritoneal fluid from women with endometriosis on implantation in the mouse model. Fertil Steril 74:41–48 - PubMed
-
- Arici A, Oral E, Bukulmez O, Duleba A, Olive DL, Jones EE. 1996. The effect of endometriosis on implantation: results from the Yale University in vitro fertilization and embryo transfer program. Fertil Steril 65:603–607 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

