A pilot study of the impact of high-frequency chest wall oscillation in chronic obstructive pulmonary disease patients with mucus hypersecretion
- PMID: 22259246
- PMCID: PMC3257955
- DOI: 10.2147/COPD.S22896
A pilot study of the impact of high-frequency chest wall oscillation in chronic obstructive pulmonary disease patients with mucus hypersecretion
Abstract
Introduction: Chronic obstructive pulmonary disease (COPD) patients with mucus hypersecretion tend to demonstrate increased frequency of infective exacerbations and a steeper slope of decline in lung function. Enhanced mucociliary clearance with high-frequency chest wall oscillation (HFCWO) devices previously used in cystic fibrosis and bronchiectasis patients may offer the opportunity for community-based, self-managed therapy to improve quality of life and lung function.
Study design and methods: A randomized controlled crossover pilot study of HFCWO compared with conventional treatment was conducted in 22 patients with moderate to severe COPD and mucus hypersecretion. Patients spent 4 weeks using an HFCWO (SmartVest(®)) device and 4 weeks in a conventional phase with a 2-week washout. Eleven patients started with HFCWO and changed to conventional treatment, whereas the other eleven patients started conventional treatment and crossed over to HFCWO.
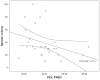
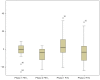
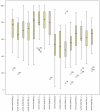
Results: The patients were elderly with a mean age of 71 (standard deviation [SD] 10) years and were at the upper end of the normal range of body mass index (25 [SD 4.2] kg/m(2)). The majority of patients had moderate to severe COPD with a mean percentage predicted forced expiratory volume in 1 second of 41 (SD 15.6) and percentage predicted forced vital capacity of 73 (SD 17.7). Baseline sputum production was negatively correlated to lung function and positively to St George's Respiratory Questionnaire. Symptom scores and St George's Respiratory Questionnaire symptom dimension improved significantly (-8, P < 0.05). Sputum production showed a declining trend in the HFCWO phase, although not reaching statistical significance. The HFCWO device was well tolerated with good reported compliance.
Conclusion: This pilot study demonstrated that patients with advanced COPD and mucus hypersecretion at increased risk of declining lung function tolerated the HFCWO treatment well, leading to improvement in quality of life and reduced symptoms.
Trial registration: ClinicalTrials.gov NCT00863616.
Keywords: bronchiectasis; sputum volume; symptom scopes.
Figures
Similar articles
-
Effects of High Frequency Chest Wall Oscillation (HFCWO) on Clinical Symptoms in COPD.Res Sq [Preprint]. 2024 Apr 11:rs.3.rs-4165729. doi: 10.21203/rs.3.rs-4165729/v1. Res Sq. 2024. PMID: 38659871 Free PMC article. Preprint.
-
Safety and effectiveness of the high-frequency chest wall oscillation vs intrapulmonary percussive ventilation in patients with severe COPD.Int J Chron Obstruct Pulmon Dis. 2018 Feb 16;13:617-625. doi: 10.2147/COPD.S145440. eCollection 2018. Int J Chron Obstruct Pulmon Dis. 2018. PMID: 29497290 Free PMC article. Clinical Trial.
-
Oscillating devices for airway clearance in people with cystic fibrosis.Cochrane Database Syst Rev. 2020 Apr 30;4(4):CD006842. doi: 10.1002/14651858.CD006842.pub5. Cochrane Database Syst Rev. 2020. PMID: 32352564 Free PMC article.
-
The effectiveness of a mobile high-frequency chest wall oscillation (HFCWO) device for airway clearance.Pediatr Pulmonol. 2020 Aug;55(8):1984-1992. doi: 10.1002/ppul.24784. Epub 2020 Apr 22. Pediatr Pulmonol. 2020. PMID: 32320537 Free PMC article. Clinical Trial.
-
Effects of High-Frequency Chest Wall Oscillation on Acute Exacerbation of Chronic Obstructive Pulmonary Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials.Int J Chron Obstruct Pulmon Dis. 2022 Nov 10;17:2857-2869. doi: 10.2147/COPD.S378642. eCollection 2022. Int J Chron Obstruct Pulmon Dis. 2022. PMID: 36381994 Free PMC article.
Cited by
-
Positive expiratory pressure therapy versus other airway clearance techniques for bronchiectasis.Cochrane Database Syst Rev. 2017 Sep 27;9(9):CD011699. doi: 10.1002/14651858.CD011699.pub2. Cochrane Database Syst Rev. 2017. PMID: 28952156 Free PMC article.
-
Effects of High Frequency Chest Wall Oscillation (HFCWO) on Clinical Symptoms in COPD.Res Sq [Preprint]. 2024 Apr 11:rs.3.rs-4165729. doi: 10.21203/rs.3.rs-4165729/v1. Res Sq. 2024. PMID: 38659871 Free PMC article. Preprint.
-
The therapeutic potential of CFTR modulators for COPD and other airway diseases.Curr Opin Pharmacol. 2017 Jun;34:132-139. doi: 10.1016/j.coph.2017.09.013. Epub 2017 Nov 10. Curr Opin Pharmacol. 2017. PMID: 29132121 Free PMC article. Review.
-
Effects of High-Frequency Chest Wall Oscillation Expectoration System on Pulmonary Rehabilitation and Cortisol Function in Patients with Severe AECOPD.Dis Markers. 2022 Jul 22;2022:3380048. doi: 10.1155/2022/3380048. eCollection 2022. Dis Markers. 2022. PMID: 35909888 Free PMC article.
-
β2-Adrenoceptor involved in smoking-induced airway mucus hypersecretion through β-arrestin-dependent signaling.PLoS One. 2014 Jun 6;9(6):e97788. doi: 10.1371/journal.pone.0097788. eCollection 2014. PLoS One. 2014. PMID: 24905583 Free PMC article.
References
-
- Mapel D, Chen JC, George D, Halbert RJ. The cost of chronic obstructive pulmonary disease and its effects on managed care. Manag Care Interface. 2004;17:61–66. - PubMed
-
- Miller RM, George D, Halbert RJ. Improving the management of chronic obstructive pulmonary disease. J Healthc Qual. 2005;27:42–47. - PubMed
-
- Sin DD, Man SFP. Pharmacotherapy for mortality reduction in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2006;3:624–629. - PubMed
-
- Niewoehner DE. The impact of severe exacerbations on quality of life and the clinical course of chronic obstructive pulmonary disease. Am J Med. 2006;119(10 suppl 1):38–45. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

