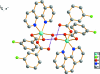
Aqua-(3-fluoro-benzoato-κO)(3-fluoro-benzoato-κO,O')(1,10-phenanthroline-κN,N')cobalt(II)
- PMID: 22259322
- PMCID: PMC3254294
- DOI: 10.1107/S1600536811051804
Aqua-(3-fluoro-benzoato-κO)(3-fluoro-benzoato-κO,O')(1,10-phenanthroline-κN,N')cobalt(II)
Abstract
In the title compound, [Co(C(7)H(4)FO(2))(2)(C(12)H(8)N(2))(H(2)O)], the Co(II) ion is coordinated by two O atoms from one 3-fluoro-benzoate (fb) ligand and one O atom from another fb ligand, two N atoms from the 1,10-phenanthroline ligand and a water mol-ecule in a distorted octa-hedral geometry. An intra-molecular O-H⋯O hydrogen bond occurs. Inter-molecular O-H⋯O hydrogen bonds link pairs of mol-ecules into centrosymmetric dimers. Weak inter-molecular C-H⋯O and C-H⋯F hydrogen bonds and π-π inter-actions between the aromatic rings [shortest centroid-centroid distance = 3.4962 (2) Å] further stabilize the crystal packing.
Figures
Similar articles
-
Aqua-(4-fluoro-benzoato-κO)bis-(1,10-phenanthroline-κN,N')manganese(II) 4-fluoro-benzoate trihydrate.Acta Crystallogr Sect E Struct Rep Online. 2011 Dec 1;67(Pt 12):m1853. doi: 10.1107/S1600536811049968. Epub 2011 Nov 30. Acta Crystallogr Sect E Struct Rep Online. 2011. PMID: 22199624 Free PMC article.
-
Aqua-bis-(3-fluoro-benzoato-κO)(1,10-phenanthroline-κN,N')copper(II).Acta Crystallogr Sect E Struct Rep Online. 2011 May 1;67(Pt 5):m564-5. doi: 10.1107/S1600536811012220. Epub 2011 Apr 13. Acta Crystallogr Sect E Struct Rep Online. 2011. PMID: 21754295 Free PMC article.
-
Diaqua-(isonicotinamide-κN)(4-meth-oxy-benzoato-κO,O')(4-meth-oxy-benzoato-κO)cobalt(II).Acta Crystallogr Sect E Struct Rep Online. 2010 Jun 16;66(Pt 7):m784-5. doi: 10.1107/S160053681002194X. Acta Crystallogr Sect E Struct Rep Online. 2010. PMID: 21587710 Free PMC article.
-
Di-μ-nicotinamide-κN:O;κO:N-bis-[aqua-bis-(4-bromo-benzoato)-κO;κO,O'-manganese(II)].Acta Crystallogr Sect E Struct Rep Online. 2011 Aug 1;67(Pt 8):m1128-9. doi: 10.1107/S1600536811028492. Epub 2011 Jul 23. Acta Crystallogr Sect E Struct Rep Online. 2011. PMID: 22090892 Free PMC article.
-
Bis(μ-3-hydroxy-benzoato-κO,O':O)bis-[aqua-(3-hydroxy-benzoato-κO,O')(1,10-phenanthroline-κN,N')lead(II)] monohydrate.Acta Crystallogr Sect E Struct Rep Online. 2007 Dec 12;64(Pt 1):m152-3. doi: 10.1107/S1600536807065075. Acta Crystallogr Sect E Struct Rep Online. 2007. PMID: 21200505 Free PMC article.
Cited by
-
Aqua-bis-(4-chloro-benzoato)-κ(2) O,O';κO-bis-(pyridine-κN)cobalt(II).Acta Crystallogr Sect E Struct Rep Online. 2013 Mar 16;69(Pt 4):m210. doi: 10.1107/S1600536813006752. Print 2013 Apr 1. Acta Crystallogr Sect E Struct Rep Online. 2013. PMID: 23634005 Free PMC article.
References
-
- Bruker (2005). APEX2, SAINT and SADABS Bruker AXS Inc., Madison, Wisconsin, USA.
-
- Miyasaka, H., Motokawa, N., Atsuumi, R., Kamo, H., Asai, Y. & Yamashita, M. (2011). Dalton Trans. 40, 673–682. - PubMed
-
- Motokawa, N., Miyasaka, H., Yamashita, M. & Dunbar, K. R. (2008). Angew. Chem. Int. Ed. Engl. 47, 7760–7763. - PubMed
-
- Sevryugina, Y., Hietsoi, O. & Petrukhina, M. A. (2007). Chem. Commun. 691, 3853–3855. - PubMed
-
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous