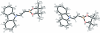9-[(E)-2-(4,4,5,5-Tetra-methyl-1,3,2-dioxa-borolan-2-yl)ethen-yl]-9H-carbazole
- PMID: 22259583
- PMCID: PMC3254436
- DOI: 10.1107/S1600536811052470
9-[(E)-2-(4,4,5,5-Tetra-methyl-1,3,2-dioxa-borolan-2-yl)ethen-yl]-9H-carbazole
Abstract
The title compound, C(20)H(22)BNO(2), is a simple olefinic compound which carries both B and N atoms that are trans to one another. The π-conjugated system of the compound is considered to be isoelectronic with 1,3-butadiene. There are two independent mol-ecules in the asymmetric unit in which the environments around the boron atoms are essentially planar (r.m.s. deviations of 0.0032 and 0.0021 Å for the BO(2)C planes). The dihedral angles of the olefinic planes with the boron planes are 5.70 (11) and 9.74 (9)°, respectively, while the dihedral angles of the olefinic planes with the carbazole planes are 19.37 (3) and 10.74 (6)°. These dihedral angles are consistent with those in 9-ethenylcarbazole and an ethenylboronic ester derivative. The N-Csp(2), B-Csp(2) and C=C bond lengths suggest that the contribution of the canonical structure can be described as N(+)=C-C=B(-).
Figures
References
-
- Altomare, A., Cascarano, G., Giacovazzo, C., Guagliardi, A., Burla, M. C., Polidori, G. & Camalli, M. (1994). J. Appl. Cryst. 27, 435.
-
- Clark, J. S., Freeman, R. P., Cacho, M., Thomas, A. W., Swallow, S. & Wilson, C. (2004). Tetrahedron Lett. 45, 8639–8642.
-
- Farrugia, L. J. (1997). J. Appl. Cryst. 30, 565.
-
- Geier, M. J., Vogels, C. M., Decken, A. & Westcott, S. A. (2009). J. Organomet. Chem. 694, 3154–3159.
-
- Rigaku (1999). NUMABS Rigaku Corporation, Tokyo, Japan.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous