Effects of the NK1 antagonist, aprepitant, on response to oral and intranasal oxycodone in prescription opioid abusers
- PMID: 22260216
- PMCID: PMC4354863
- DOI: 10.1111/j.1369-1600.2011.00419.x
Effects of the NK1 antagonist, aprepitant, on response to oral and intranasal oxycodone in prescription opioid abusers
Abstract
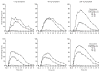
Pre-clinical studies suggest that the neurokinin-1 (NK1) receptor may modulate the response to opioids, with NK(1) inactivation leading to decreased opioid reinforcement, tolerance and withdrawal. Aprepitant is a selective NK1 antagonist currently marketed for clinical use as an anti-emetic. This 6-week in-patient study used a randomized, double-blind, double-dummy, within-subject, crossover design. Subjects (n = 8; 6 male/2 female) were healthy, adult volunteers who provided subjective and objective evidence of current prescription opioid abuse (without physical dependence) and underwent careful medical and psychiatric screening. Fifteen experimental conditions, consisting of one aprepitant dose (0, 40 and 200 mg, p.o. given as a 2-hour pre-treatment) in combination with one oxycodone dose [placebo, oral (20 and 40 mg/70 kg) and intranasal (15 and 30 mg/70 kg)], were examined. Sessions were conducted at least 48-hour apart and multi-dimensional measures were collected repeatedly throughout the 6-hour session duration. Oxycodone, by both routes of administration, produced significant dose-related effects on the predicted measures (e.g. subjective measures of abuse liability, respiratory depression and miosis). Pre-treatment with aprepitant (200 mg) significantly enhanced ratings of oxycodone subjective effects related to euphoria and liking and doubled the street value estimates for the highest test doses of oxycodone by both routes. Some objective measures (respiratory function, observer-rated opioid agonist effects) were similarly enhanced by pre-treatment with the highest dose of aprepitant. All dose combinations were safely tolerated. These findings are discussed in the context of the potential utility of NK1 antagonists in the treatment of opioid use disorders.
Trial registration: ClinicalTrials.gov NCT00999544.
© 2012 The Authors, Addiction Biology © 2012 Society for the Study of Addiction.
Figures



Similar articles
-
Evaluation of tradipitant, a selective NK1 antagonist, on response to oxycodone in humans.Psychopharmacology (Berl). 2021 Jul;238(7):1857-1866. doi: 10.1007/s00213-021-05814-x. Epub 2021 May 14. Psychopharmacology (Berl). 2021. PMID: 33988725 Free PMC article. Clinical Trial.
-
Opioid-like effects of the neurokinin 1 antagonist aprepitant in patients maintained on and briefly withdrawn from methadone.Am J Drug Alcohol Abuse. 2013 Mar;39(2):86-91. doi: 10.3109/00952990.2012.762372. Am J Drug Alcohol Abuse. 2013. PMID: 23421568 Free PMC article. Clinical Trial.
-
Intranasal buprenorphine alone and in combination with naloxone: Abuse liability and reinforcing efficacy in physically dependent opioid abusers.Drug Alcohol Depend. 2016 May 1;162:190-8. doi: 10.1016/j.drugalcdep.2016.03.005. Epub 2016 Mar 14. Drug Alcohol Depend. 2016. PMID: 27012435 Free PMC article. Clinical Trial.
-
Aprepitant: an oral NK1 antagonist for the prevention of nausea and vomiting induced by highly emetogenic chemotherapy.Drugs Today (Barc). 2004 Oct;40(10):853-63. doi: 10.1358/dot.2004.40.10.863745. Drugs Today (Barc). 2004. PMID: 15605119 Review.
-
Aprepitant--a novel NK1-receptor antagonist.Expert Opin Pharmacother. 2003 Dec;4(12):2279-96. doi: 10.1517/14656566.4.12.2279. Expert Opin Pharmacother. 2003. PMID: 14640927 Review.
Cited by
-
Intranasal oxycodone self-administration in non-dependent opioid abusers.Exp Clin Psychopharmacol. 2012 Aug;20(4):310-7. doi: 10.1037/a0028327. Epub 2012 Jun 11. Exp Clin Psychopharmacol. 2012. PMID: 22686495 Free PMC article. Clinical Trial.
-
Functional evaluation of NK1 antagonism on cue reactivity in opiate dependence; An fMRI study.Drug Alcohol Depend. 2021 Apr 1;221:108564. doi: 10.1016/j.drugalcdep.2021.108564. Epub 2021 Jan 29. Drug Alcohol Depend. 2021. PMID: 33548897 Free PMC article.
-
The Useage of Opioids and their Adverse Effects in Gastrointestinal Practice: A Review.Middle East J Dig Dis. 2013 Jan;5(1):5-16. Middle East J Dig Dis. 2013. PMID: 24829664 Free PMC article. Review.
-
Hydrocodone, Oxycodone, and Morphine Metabolism and Drug-Drug Interactions.J Pharmacol Exp Ther. 2023 Nov;387(2):150-169. doi: 10.1124/jpet.123.001651. Epub 2023 Sep 7. J Pharmacol Exp Ther. 2023. PMID: 37679047 Free PMC article. Review.
-
Substance use disorders: psychoneuroimmunological mechanisms and new targets for therapy.Pharmacol Ther. 2013 Aug;139(2):289-300. doi: 10.1016/j.pharmthera.2013.04.011. Epub 2013 Apr 28. Pharmacol Ther. 2013. PMID: 23631821 Free PMC article. Review.
References
-
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;54:561–571. - PubMed
-
- Birklein F, Schmelz M. Neuropeptides, neurogenic inflammation and complex regional pain syndrome (CRPS) Neurosci Lett. 2008;437:199–2002. - PubMed
-
- Costa PT, McCrae RR. The NEO Personality Inventory Manual. Psychological Assessment Resources; Odessa, Florida: 1985.
-
- Dando TM, Perry CM. Aprepitant: A review of its use in the prevention of chemotherapy-induced nausea and vomiting. Drugs. 2004;64:777–794. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

