EGFR ligands exhibit functional differences in models of paracrine and autocrine signaling
- PMID: 22260327
- PMCID: PMC3962550
- DOI: 10.3109/08977194.2011.649918
EGFR ligands exhibit functional differences in models of paracrine and autocrine signaling
Abstract
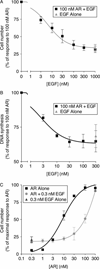
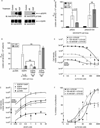
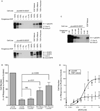
Epidermal growth factor (EGF) family peptides are ligands for the EGF receptor (EGFR). Here, we elucidate functional differences among EGFR ligands and mechanisms underlying these distinctions. In 32D/EGFR myeloid and MCF10A breast cells, soluble amphiregulin (AR), transforming growth factor alpha (TGFα), neuregulin 2 beta, and epigen stimulate greater EGFR coupling to cell proliferation and DNA synthesis than do EGF, betacellulin, heparin-binding EGF-like growth factor, and epiregulin. EGF competitively antagonizes AR, indicating that its functional differences reflect dissimilar intrinsic activity at EGFR. EGF stimulates much greater phosphorylation of EGFR Tyr1045 than does AR. Moreover, the EGFR Y1045F mutation and z-cbl dominant-negative mutant of the c-cbl ubiquitin ligase potentiate the effect of EGF but not of AR. Both EGF and AR stimulate phosphorylation of EGFR Tyr992. However, the EGFR Y992F mutation and phospholipase C gamma inhibitor U73122 reduce the effect of AR much more than that of EGF. Expression of TGFα in 32D/EGFR cells causes greater EGFR coupling to cell proliferation than does expression of EGF. Moreover, expression of EGF in 32D/EGFR cells causes these cells to be largely refractory to stimulation with soluble EGF. Thus, EGFR ligands are functionally distinct in models of paracrine and autocrine signaling and EGFR coupling to biological responses may be specified by competition among functionally distinct EGFR ligands.
Conflict of interest statement
Figures

References
-
- Baulida J, Kraus MH, Alimandi M, Di Fiore PP, Carpenter G. All ErbB receptors other than the epidermal growth factor receptor are endocytosis impaired. J Biol Chem. 1996;271:5251–5257. - PubMed
-
- Citri A, Yarden Y. EGF-ERBB signalling: Towards the systems level. Nat Rev Mol Cell Biol. 2006;7:505–516. - PubMed
-
- Duan L, Miura Y, Dimri M, Majumder B, Dodge IL, Reddi AL, Ghosh A, Fernandes N, Zhou P, Mullane-Robinson K, Rao N, Donoghue S, Rogers RA, Bowtell D, Naramura M, Gu H, Band V, Band H. Cbl-mediated ubiquitinylation is required for lysosomal sorting of epidermal growth factor receptor but is dispensable for endocytosis. J Biol Chem. 2003;278:28950–28960. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
