MicroRNA-24 regulates cardiac fibrosis after myocardial infarction
- PMID: 22260784
- PMCID: PMC3822985
- DOI: 10.1111/j.1582-4934.2012.01523.x
MicroRNA-24 regulates cardiac fibrosis after myocardial infarction
Abstract
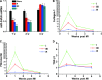
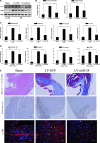
Cardiac fibrosis after myocardial infarction (MI) has been identified as a key factor in the development of heart failure. Although dysregulation of microRNA (miRNA) is involved in various pathophysiological processes in the heart, the role of miRNA in fibrosis regulation after MI is not clear. Previously we observed the correlation between fibrosis and the miR-24 expression in hypertrophic hearts, herein we assessed how miR-24 regulates fibrosis after MI. Using qRT-PCR, we showed that miR-24 was down-regulated in the MI heart; the change in miR-24 expression was closely related to extracellular matrix (ECM) remodelling. In vivo, miR-24 could improve heart function and attenuate fibrosis in the infarct border zone of the heart two weeks after MI through intramyocardial injection of Lentiviruses. Moreover, in vitro experiments suggested that up-regulation of miR-24 by synthetic miR-24 precursors could reduce fibrosis and also decrease the differentiation and migration of cardiac fibroblasts (CFs). TGF-β (a pathological mediator of fibrotic disease) increased miR-24 expression, overexpression of miR-24 reduced TGF-β secretion and Smad2/3 phosphorylation in CFs. By performing microarray analyses and bioinformatics analyses, we found furin to be a potential target for miR-24 in fibrosis (furin is a protease which controls latent TGF-β activation processing). Finally, we demonstrated that protein and mRNA levels of furin were regulated by miR-24 in CFs. These findings suggest that miR-24 has a critical role in CF function and cardiac fibrosis after MI through a furin-TGF-β pathway. Thus, miR-24 may be used as a target for treatment of MI and other fibrotic heart diseases.
© 2012 The Authors Journal of Cellular and Molecular Medicine © 2012 Foundation for Cellular and Molecular Medicine/Blackwell Publishing Ltd.
Figures




References
-
- van den Borne SW, Diez J, Blankesteijn WM, et al. Myocardial remodeling after infarction: the role of myofibroblasts. Nat Rev Cardiol. 2010;7:30–7. - PubMed
-
- Tomasek JJ, Gabbiani G, Hinz B, et al. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev. 2002;3:349–63. - PubMed
-
- Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–5. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

