Accelerating control of pertussis in England and Wales
- PMID: 22260989
- PMCID: PMC3381681
- DOI: 10.3201/eid1801.110784
Accelerating control of pertussis in England and Wales
Abstract
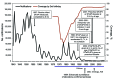
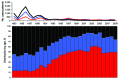
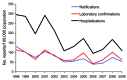
Results of an accelerated pertussis vaccination schedule for infants introduced in 1990 in England and Wales were examined. Earlier scheduling and sustained high vaccine coverage resulted in fewer reported cases of pertussis among infants, reinforcing the World Health Organization drive for on-time completion of the infant vaccination schedule. As determined by using the screening method, the first dose of vaccine was 61.7% effective in infants <6 months of age, and effectiveness increased with subsequent doses. Three doses of a good whole-cell pertussis vaccine were 83.7% effective in children 10-16 years of age; a preschool booster vaccination further reduced pertussis incidence in children <10 years of age. As in other industrialized countries, surveillance data during 1998-2009 showed that pertussis in England and Wales mainly persists in young infants (i.e., <3 months of age), teenagers, and adults. Future vaccine program changes may be beneficial, but additional detail is required to inform such decisions.
Figures
References
-
- Miller E, Vurdien JE, White JM. The epidemiology of pertussis in England and Wales. Commun Dis Rep CDR Rev. 1992;2:R152–4. - PubMed
-
- Andrews N, Stowe J, Wise L, Miller E. Post-licensure comparison of the safety profile of diphtheria/tetanus/whole cell pertussis/haemophilus influenza type b vaccine and a 5-in-1diphtheria/tetanus/acellular pertussis/haemophilus influenza type b/polio vaccine in the United Kingdom. Vaccine. 2010;28:7215–20. 10.1016/j.vaccine.2010.08.062 - DOI - PubMed
-
- Health Protection Agency. Vaccination coverage statistics for children aged up to five years in the United Kingdom: July to December 2009. Health Protection Report, vol 4, no 12; 2010 Mar 26 [cited 2011 May 10]. http://www.hpa.org.uk/hpr/archives/2010/hpr1210.pdf
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
